推薦產品
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to beige
運輸包裝
dry ice
儲存溫度
−20°C
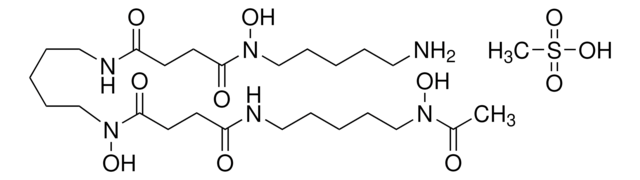
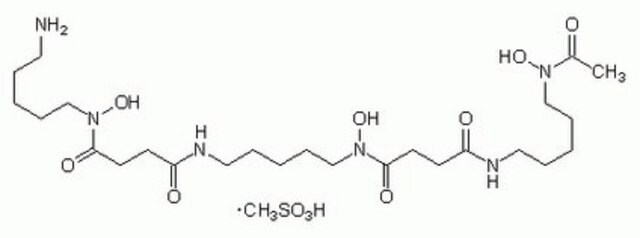
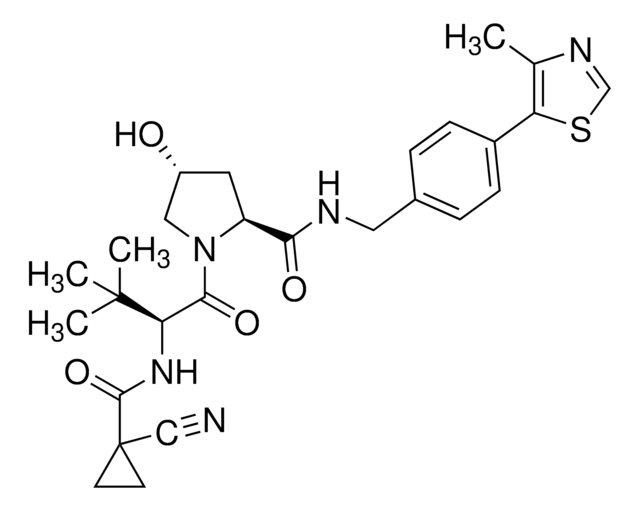
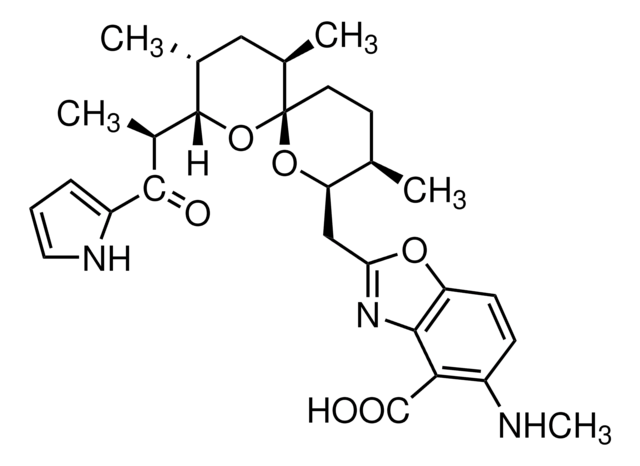
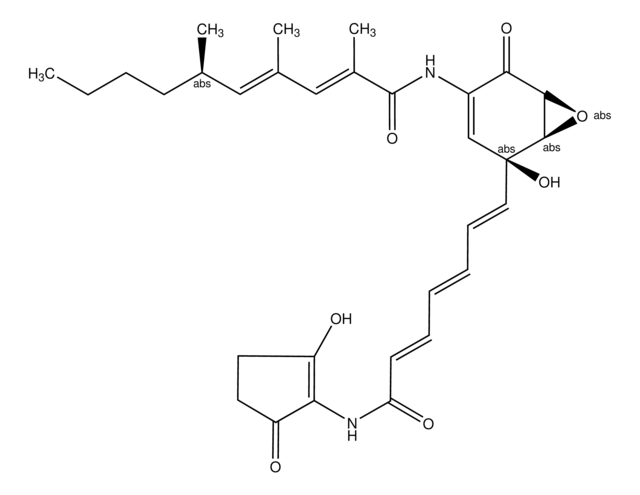
SMILES 字串
S1C(C2N(C(=O)C(NC(=O)C(COC(=O)C(N(C(=O)C(N(C(=O)C(NC(=O)C(COC(=O)C(N(C2=O)C)C(C)C)NC(=O)c5nc6c(nc5)cccc6)C)C)C1)C)C(C)C)NC(=O)c3nc4c(nc3)cccc4)C)C)SC
InChI
1S/C51H64N12O12S2/c1-25(2)38-49(72)74-22-36(59-42(65)34-21-53-30-17-13-15-19-32(30)57-34)44(67)55-28(6)46(69)63(10)40-48(71)62(9)39(26(3)4)50(73)75-23-35(58-41(64)33-20-52-29-16-12-14-18-31(29)56-33)43(66)54-27(5)45(68)60(7)37(47(70)61(38)8)24-77-51(40)76-11/h12-21,25-28,35-40,51H,22-24H2,1-11H3,(H,54,66)(H,55,67)(H,58,64)(H,59,65)
InChI 密鑰
AUJXLBOHYWTPFV-UHFFFAOYSA-N
應用
生化/生理作用
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Repr. 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務