推薦產品
化驗
≥99% (TLC)
形狀
powder
mp
157-159 °C (lit.)
溶解度
water: 50 mg/mL, clear, colorless
儲存溫度
2-8°C
SMILES 字串
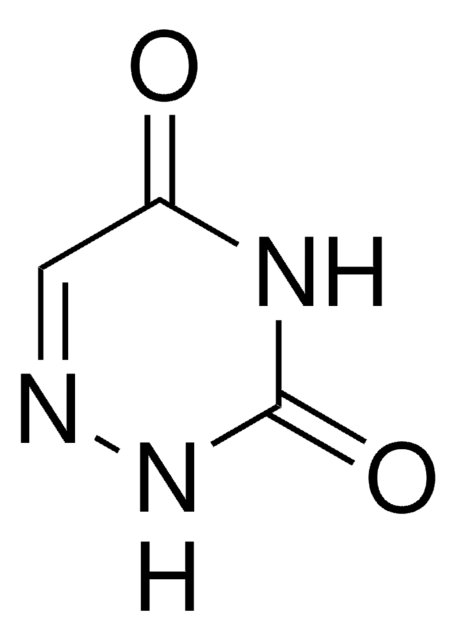
OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N2N=CC(=O)NC2=O
InChI
1S/C8H11N3O6/c12-2-3-5(14)6(15)7(17-3)11-8(16)10-4(13)1-9-11/h1,3,5-7,12,14-15H,2H2,(H,10,13,16)/t3-,5-,6-,7-/m1/s1
InChI 密鑰
WYXSYVWAUAUWLD-SHUUEZRQSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
6-氮杂尿苷是嘧啶核苷类似物。
應用
6-氮杂尿苷已用于:
- 用于尿苷一磷酸合酶(UMPS)活性测定
- 作为抗病毒药物,研究其对足口病病毒的抑制效应和细胞毒活性
- 筛选抗隐孢子虫活性
- 对HeLa细胞进行预处理,以研究捕获5-溴尿苷5′-三磷酸(BrUTP)前和捕获过程中抑制细胞尿苷合成的效应
- 用作参照化合物,比较其对病毒宿主细胞株的抗病毒活性和细胞毒活性
6-氮杂尿苷(AzUrd)阻止乳清酸转化为UMP,并用于抗病毒研究。
生化/生理作用
6-氮杂尿苷是一种前药,在转化为6-氮杂-UMP后会抑制尿苷单磷酸合酶(UMPS)。它是一种广谱的抗代谢药。它干扰嘧啶的生物合成并影响细胞核酸水平。它被认为是一种抗肿瘤代谢物。它抑制基孔肯雅病毒、塞姆利基森林病毒和人类冠状病毒等RNA病毒。它显著抑制隐孢子虫寄生虫的生长。
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
S Bhasin et al.
The American journal of physiology, 243(3), E234-E239 (1982-09-01)
Ketonemic states complicating late pregnancy are accompanied by lower brain weights in the newborn. Potential mechanisms whereby ketone bodies might inhibit cell proliferation were therefore examined in the fetal rat brain slice by measuring their impact on the de novo
Takashi Kobayashi et al.
Physical chemistry chemical physics : PCCP, 12(19), 5140-5148 (2010-05-07)
Excited state characteristics of 6-azauridine (6AUd), which is known as a medicine against psoriasis and neoplastic, were investigated with laser plash photolysis, time-resolved thermal lensing, and near IR single photon counting method. The triplet-triplet absorption spectrum of 6AUd was observed
Enhanced inhibition of foot-and-mouth disease virus by combinations of porcine interferon-alpha and antiviral agents
Kim SM, et al.
Antiviral research, 96(2), 213-220 (2012)
Krzysztof Pyrc et al.
Antimicrobial agents and chemotherapy, 50(6), 2000-2008 (2006-05-26)
Human coronavirus NL63 (HCoV-NL63), a recently discovered member of the Coronaviridae family, has spread worldwide and is associated with acute respiratory illness in young children and elderly and immunocompromised persons. Further analysis of HCoV-NL63 pathogenicity seems warranted, in particular because
Donald F Smee et al.
Journal of virological methods, 246, 51-57 (2017-04-01)
Studies were conducted to determine the performance of four dyes in assessing antiviral activities of compounds against three RNA viruses with differing cytopathogenic properties. Dyes included alamarBlue
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務