推薦產品
等級
pharmaceutical primary standard
API 家族
quinidine
製造商/商標名
EDQM
mp
212-214 °C (dec.) (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
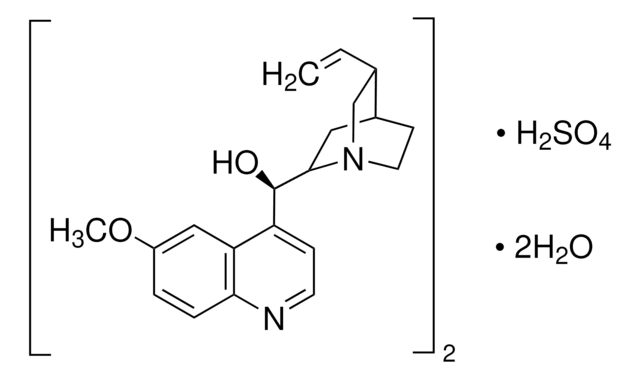
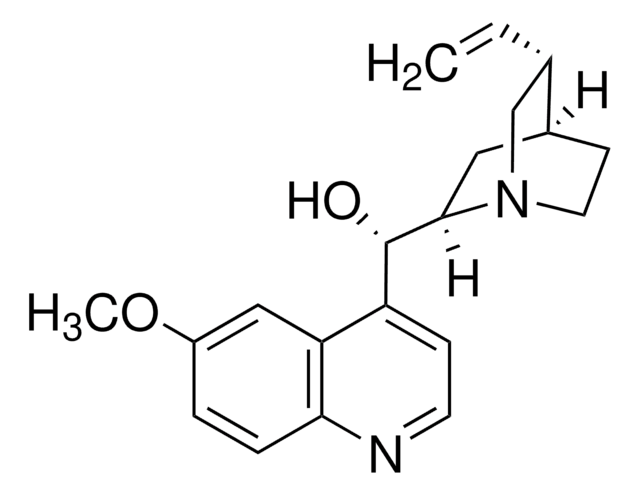
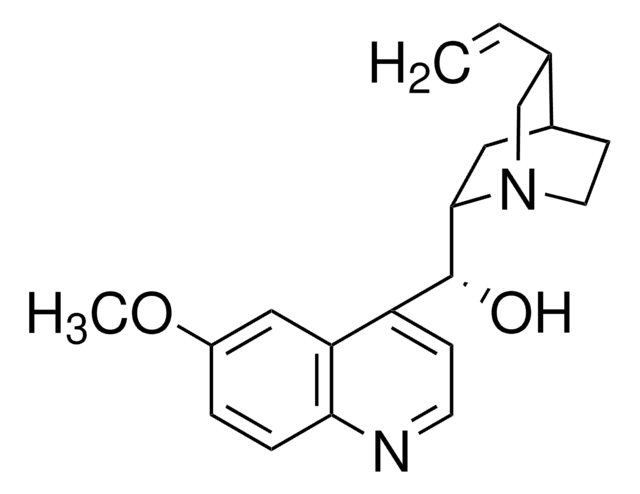
SMILES 字串
[H]O[H].[H]O[H].OS(O)(=O)=O.[H][C@]1(CN2CCC1C[C@]2([H])[C@@H](O)c3ccnc4ccc(OC)cc34)C=C.[H][C@]5(CN6CCC5C[C@]6([H])[C@@H](O)c7ccnc8ccc(OC)cc78)C=C
InChI
1S/2C20H24N2O2.H2O4S.2H2O/c2*1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18;1-5(2,3)4;;/h2*3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3;(H2,1,2,3,4);2*1H2/t2*13-,14?,19-,20+;;;/m11.../s1
InChI 密鑰
ZHNFLHYOFXQIOW-WFTMRWCJSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Quinidine sulfate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
IA 类抗心律失常药;钾通道阻断剂。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Motoya Suzuki et al.
Xenobiotica; the fate of foreign compounds in biological systems, 44(11), 1039-1045 (2014-05-21)
1. This study was aimed to characterize gastrointestinal absorption of digoxin using wild-type (WT) and multidrug resistance protein 1a [mdr1a; P-glycoprotein (P-gp)] knockout (-/-) rats. 2. In WT rats, the area under the plasma concentration-time curve (AUC) of oral digoxin
Yuki Ogawa et al.
European journal of pediatrics, 174(4), 509-518 (2014-09-25)
This study aimed to determine the population pharmacokinetics of doxapram in low-birth-weight (LBW) infants. A total of 92 serum concentration measurements that were obtained from 34 Japanese neonates were analyzed using nonlinear mixed-effect modeling (NONMEM). Estimates generated by NONMEM indicated
Maria Addolorata Saracino et al.
Journal of pharmaceutical and biomedical analysis, 95, 61-67 (2014-03-19)
A rapid and reliable analytical method has been developed to quantify the melatonergic antidepressant agomelatine in three matrices, and namely saliva, plasma and dried blood spots. The method is based on the use of liquid chromatography with fluorimetric detection exploiting
Mikiko Tsukimoto et al.
Biopharmaceutics & drug disposition, 36(1), 15-33 (2014-09-30)
Aliskiren is a substrate for P-glycoprotein (P-gp) and is metabolized via cytochrome P450 3A4 (CYP3A4). The aim of the present study was to assess whether P-gp influenced the pharmacokinetics of aliskiren and also if drug-drug interactions (DDIs) mediated through P-gp
Laura Kervezee et al.
The AAPS journal, 16(5), 1029-1037 (2014-06-12)
Nearly all bodily processes exhibit circadian rhythmicity. As a consequence, the pharmacokinetic and pharmacodynamic properties of a drug may also vary with time of day. The objective of this study was to investigate diurnal variation in processes that regulate drug
條款
LC/MS/MS Analysis of Drugs in Plasma on Ascentis® Express C18 after Extraction using SPME LC Tips
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務