推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to BP 277
traceable to Ph. Eur. P0270000
traceable to USP 1496008
API 家族
papaverine
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
food and beverages
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-30°C
SMILES 字串
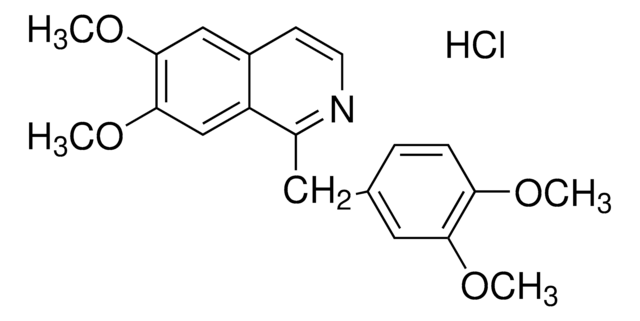
COC1=C(OC)C=C(C(CC2=CC(OC)=C(OC)C=C2)=NC=C3)C3=C1.Cl
InChI
1S/C20H21NO4.ClH/c1-22-17-6-5-13(10-18(17)23-2)9-16-15-12-20(25-4)19(24-3)11-14(15)7-8-21-16;/h5-8,10-12H,9H2,1-4H3;1H
InChI 密鑰
UOTMYNBWXDUBNX-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Papaverine hydrochloride is the hydrochloride salt of an opiate derivative called papaverine, a benzylisoquinoline compound. It has been found to relax smooth muscles of blood vessels and is used in the management of occlusivity and spasticity of pulmonary, coronary and peripheral arteries.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
Papaverine hydrochloride is used as a pharmaceutical reference standard in the determination of the analyte in pharmaceutical formulations by chromatographic and spectrophotometric techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生化/生理作用
Smooth muscle relaxant and cerebral vasodilator; phosphodiesterase inhibitor.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA9001 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Simultaneous determination of dipyrone and papaverine in pharmaceutical formulation using PLS regression and UV spectrophotometry
do Nascimento AP, et al.
Analytical Letters, 40(5), 975-986 (2007)
The effect of intravenous papaverine hydrochloride on the cerebral circulation
Jayne H W, et al
The Journal of Clinical Investigation, 31(1), 111-114 (1952)
Spectrophotometric determination of meclozine HCl and papaverine HCl in their pharmaceutical formulations
Abdel-Ghani NT, et al.
Journal of Pharmaceutical and Biomedical Analysis, 28(2), 373-378 (2002)
Alaa El-Gindy
Farmaco (Societa chimica italiana : 1989), 60(9), 745-753 (2005-07-19)
Three methods are developed for the simultaneous determination of diprophylline (DP), phenobarbitone (PH) and papaverine hydrochloride (PP). The chromatographic method depends on a high performance liquid chromatographic (HPLC) separation on a reversed-phase C18 column with a mobile phase consisting of
Simultaneous assay of ephedrine hydrochloride, theophylline, papaverine hydrochloride and hydroxyzine hydrochloride in tablets using RP-LC
Boberi B, et al.
Journal of Pharmaceutical and Biomedical Analysis, 21(1), 15-22 (1999)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務