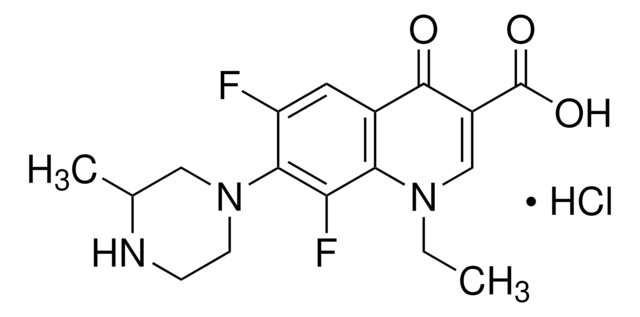
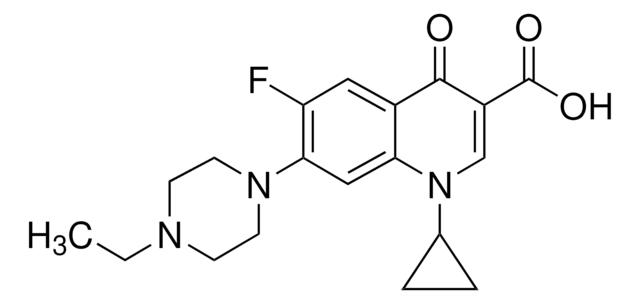
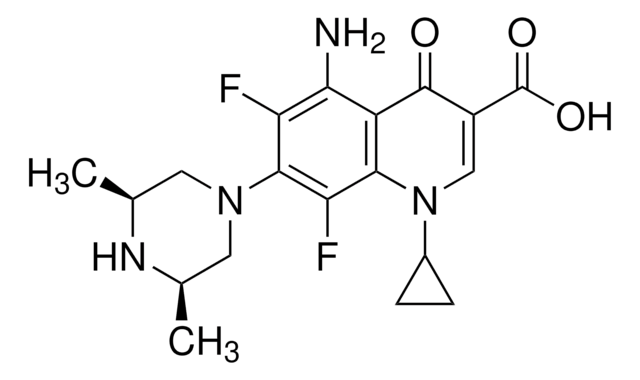
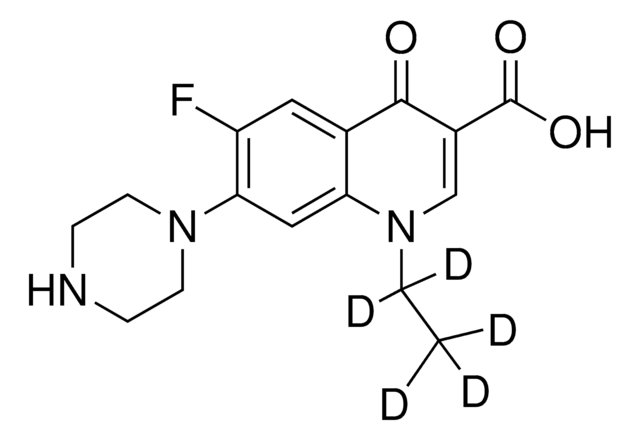
推薦產品
等級
analytical standard
品質等級
agency
EPA 1694
化驗
≥98% (TLC)
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
clinical testing
形式
neat
儲存溫度
2-8°C
SMILES 字串
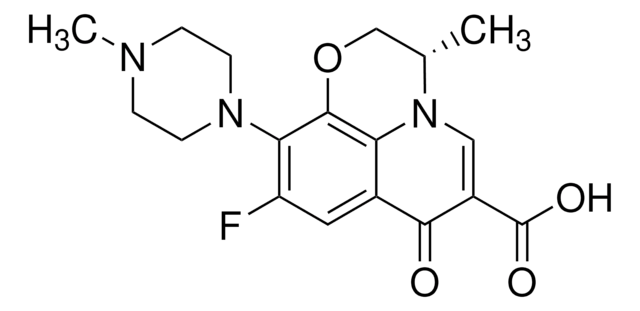
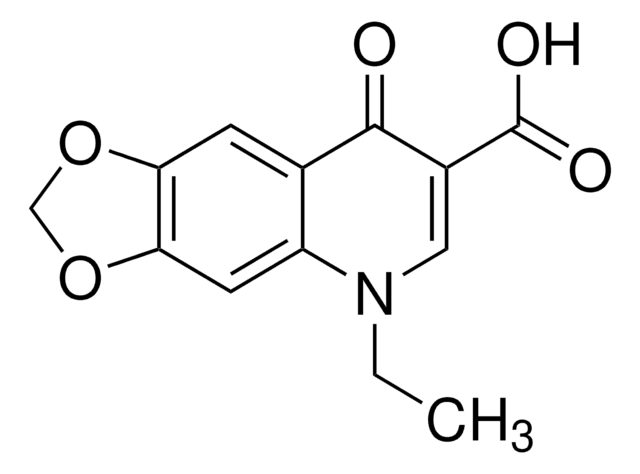
CCN1C=C(C(O)=O)C(=O)c2cc(F)c(cc12)N3CCNCC3
InChI
1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23)
InChI 密鑰
OGJPXUAPXNRGGI-UHFFFAOYSA-N
基因資訊
human ... CYP1A2(1544)
rat ... Gabra1(29705)
尋找類似的產品? 前往 產品比較指南
一般說明
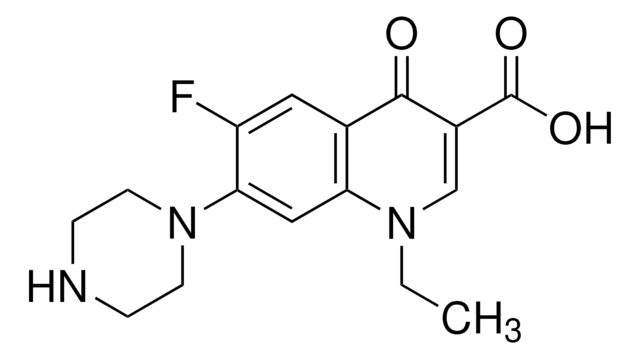
化学结构式:氟喹诺酮类
诺氟沙星 (Norfloxacin) 是一种广谱抗菌药物,对多种革兰氏阳性菌和阴性菌均有抗菌活性。
應用
参考产品的检验报告,了解更多关于合适仪器技术的信息。联系技术服务部寻求进一步支持。
诺氟沙星可作为标准品使用荧光分光光度法测定人血清样品中的诺氟沙星。
生化/生理作用
诺氟沙星通过干扰 ATP 诱导转换 DNA 与 DNA 旋转酶(拓扑异构酶)复合物的结构转换而阻断 DNA 复制。
操作方式:抑制细菌 DNA 复制
抗菌谱:革兰氏阴性菌; 对革兰氏阳性菌效果较差
操作方式:抑制细菌 DNA 复制
抗菌谱:革兰氏阴性菌; 对革兰氏阳性菌效果较差
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Fluorescence reaction and complexation equilibria between norfloxacin and aluminium (III) ion in chloride medium
Djurdjevic.TP, et al.
Analytica Chimica Acta, 300, 253-259 (1995)
L M Cavaco et al.
Journal of clinical microbiology, 47(9), 2751-2758 (2009-07-03)
Fluoroquinolone resistance in members of the Enterobacteriaceae family is mostly due to mutations in the quinolone resistance-determining regions of the topoisomerase genes. However, transferable genes encoding quinolone resistance have recently been described. The current methods for susceptibility testing are not
Deepika Sharma et al.
European journal of medicinal chemistry, 44(6), 2347-2353 (2008-10-15)
In the present study, we have synthesized 2-(substituted phenyl)-1H-imidazole (1-12) and (substituted phenyl)-[2-(substituted phenyl)-imidazol-1-yl]-methanone (13-26) analogues and screened them for their antimicrobial activity against gram positive, gram negative and fungal species. The results of antibacterial study indicated that compounds 15
Anita Reinhardt et al.
Antimicrobial agents and chemotherapy, 51(4), 1341-1350 (2007-01-31)
Intubated patients frequently become colonized by Pseudomonas aeruginosa, which is subsequently responsible for ventilator-associated pneumonia. This pathogen readily acquires resistance against available antimicrobials. Depending on the resistance mechanism selected for, resistance might either be lost or persist after removal of
Lauren Becnel Boyd et al.
Antimicrobial agents and chemotherapy, 53(1), 229-234 (2008-10-08)
Fluoroquinolones are some of the most prescribed antibiotics in the United States. Previously, we and others showed that the fluoroquinolones exhibit a class effect with regard to the CLSI-established breakpoints for resistance, such that decreased susceptibility (i.e., an increased MIC)
文章
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務