全部照片(1)
About This Item
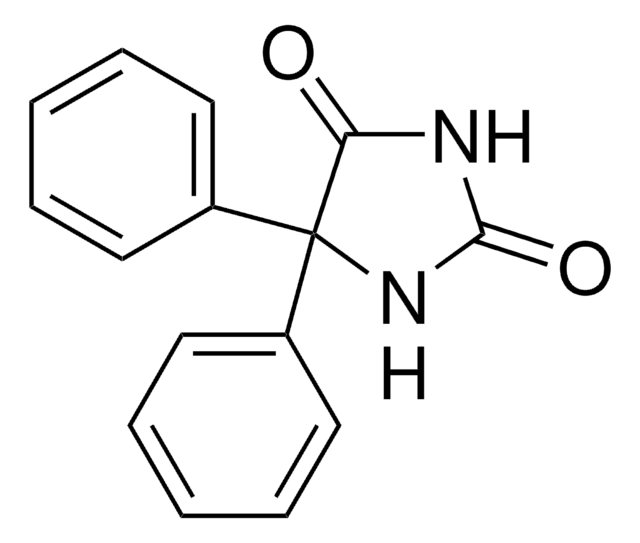
經驗公式(希爾表示法):
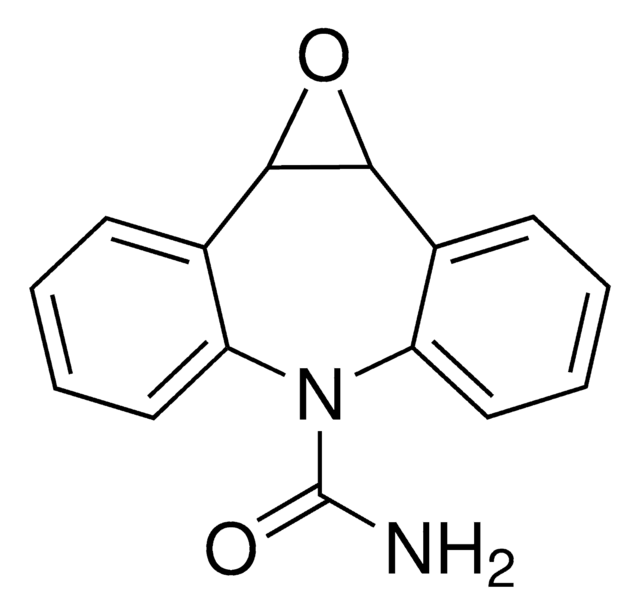
C15H12N2O2
CAS號碼:
分子量::
252.27
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
analytical standard
品質等級
化驗
≥98% (HPLC)
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
forensics and toxicology
pharmaceutical (small molecule)
veterinary
形式
neat
儲存溫度
−20°C
SMILES 字串
NC(=O)N1c2ccccc2C3OC3c4ccccc14
InChI
1S/C15H12N2O2/c16-15(18)17-11-7-3-1-5-9(11)13-14(19-13)10-6-2-4-8-12(10)17/h1-8,13-14H,(H2,16,18)
InChI 密鑰
ZRWWEEVEIOGMMT-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
卡马西平10,11-环氧化物是药物卡马西平的代谢产物。
應用
卡马西平10,11-环氧化物可用作测试材料,用于荧光HDAC活性测定法以定量组蛋白脱乙酰酶(HDAC)抑制。
有关合适仪器技术的更多信息,请参考产品′s分析证书。如需进一步支持,请联系技术服务。
生化/生理作用
卡马西平的第一代谢产物
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Ana Fortuna et al.
Analytical and bioanalytical chemistry, 397(4), 1605-1615 (2010-04-20)
For the first time, a simple, selective and accurate high-performance liquid chromatography method with ultraviolet detection was developed and validated to quantify simultaneously three structurally related antiepileptic drugs; carbamazepine, oxcarbazepine, and the recently launched eslicarbazepine acetate and their main metabolites
Sigrid Mennickent et al.
Journal of separation science, 32(9), 1454-1458 (2009-03-31)
An instrumental planar chromatographic (HPTLC) method for quantification of carbamazepine in human serum was developed using liquid-liquid extraction with dichloromethane, fluorescence activation with perchloric acid 60%/ethanol/water (1:1:1, v/v) and fluorescence detection. Planar chromatographic separation was performed on precoated silica gel
Terumitsu Yoshida et al.
Journal of pharmaceutical and biomedical analysis, 41(4), 1386-1390 (2006-04-07)
This study developed a simple method for the simultaneous determination of zonisamide (ZNS), carbamazepine (CBZ) and its active metabolite, carbamazepine-10,11-epoxide (CBZE) in infant serum using reversed-phase high-performance liquid chromatograph (HPLC). The method involves a single-step protein precipitation procedure that uses
Kyoung-Ah Kim et al.
European journal of clinical pharmacology, 61(4), 275-280 (2005-05-26)
Carbamazepine (CBZ) undergoes biotransformation by CYP3A4 and CYP2C8, and glucuronide conjugation. There has been no clear demonstration to reveal the role of glucuronidation in the disposition of CBZ. We evaluated the effect of probenecid, a UDP-glucuronosyltransferase inhibitor, on the pharmacokinetics
Andreas S Beutler et al.
Life sciences, 76(26), 3107-3115 (2005-04-27)
Carbamazepine (CBZ) is a common antiepileptic drug (AED) that acts through multiple mechanisms including blockade and potentiation of cation channels and modulation of neurotransmitter levels. Whether it affects any component of the gene transcription machinery is unknown. Histone deacetylases (HDAC)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務