推薦產品
等級
puriss. p.a.
品質等級
化驗
98%
形狀
crystals
燃燒殘留物
≤0.08% (as SO4)
mp
180-183 °C (dec.) (lit.)
負離子痕跡
sulfate (SO42-): ≤100 mg/kg
正離子痕跡
Ca: ≤50 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
官能基
amine
hydrazine
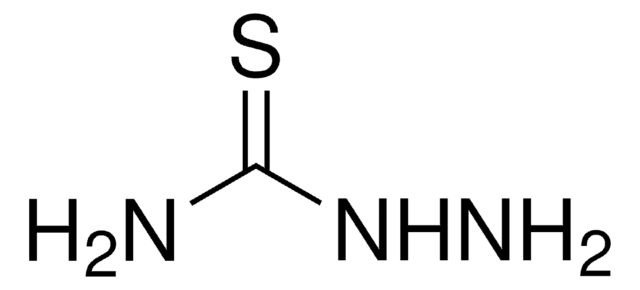
thiourea
SMILES 字串
NNC(N)=S
InChI
1S/CH5N3S/c2-1(5)4-3/h3H2,(H3,2,4,5)
InChI 密鑰
BRWIZMBXBAOCCF-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
硫代氨基脲是一种对环境有毒的化合物,通常来源于硫脲。螯合微量金属的能力提高了它对某些肿瘤、原生动物、流行性感冒、杀虫剂和杀真菌剂的生物活性。它是一种使用广泛的金属络合剂,用于涉及脂肪族或芳香族醛、酮和多糖表征的各个领域。
應用
硫代氨基脲已被用作分析试剂,用于通过比色法测量完整玉米植株根系中尿素的净高亲和力吸收。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Oral - Aquatic Chronic 3
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Electrochemical determination of thiosemicarbazide using the glassy carbon electrode modified with multi-walled carbon nanotubes
Faizbakhsh N and Safari Z
Advances in Nanochemistry, 1(2), 52-55 (2019)
Hong-Jia Zhang et al.
European journal of medicinal chemistry, 46(9), 4702-4708 (2011-08-06)
A series of novel chalcone thiosemicarbazide derivatives (4a-4x) have been designed, synthesized, structurally determined, and their biological activities were also evaluated as potential EGFR kinase inhibitors. All the synthesized compounds are first reported. Among the compounds, compound 4r showed the
José Ruiz et al.
Inorganic chemistry, 52(2), 974-982 (2013-01-11)
A series of new organoiridium(III) complexes [Ir(N-C)(2)(N-S)]Cl (HN-C = 2-phenylpyridine (Hppy), N-S = methyl thiosemicarbazide (1), phenyl thiosemicarbazide (2) and naphtyl thiosemicarbazide (3)) have been synthesized and characterized. The crystal structure of (1) has been established by X-ray diffraction, showing
Pedro I da S Maia et al.
Inorganic chemistry, 51(3), 1604-1613 (2012-01-12)
Na[AuCl(4)]·2H(2)O reacts with tridentate thiosemicarbazide ligands, H(2)L1, derived from N-[N',N'-dialkylamino(thiocarbonyl)]benzimidoyl chloride and thiosemicarbazides under formation of air-stable, green [AuCl(L1)] complexes. The organic ligands coordinate in a planar SNS coordination mode. Small amounts of gold(I) complexes of the composition [AuCl(L3)] are
Juan Liu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 93, 245-249 (2012-04-10)
A simple and highly selective colorimetric sensor (L1) bearing thiosemicarbazide moiety as binding site and nitrophenyl moiety as signal group were synthesized. Sensor L1 showed great colorimetric single selectivity and high sensitivity for mercury cation in DMSO and DMSO/H(2)O binary
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務