324521
Eeyarestatin I
A cell-permeable oxo-imidazolidinyl-hydroxyurea that localizes to ER, where it interacts with AAA ATPase p97 via its nitrofuran-containing moiety, without exhibiting affinity toward Hsp70 / ATPase NSF
同義詞:
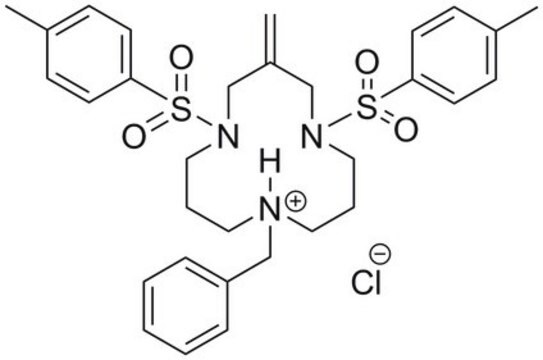
Eeyarestatin I, 1-(4-Chloro-phenyl)-3-(3-(4-chloro-phenyl)-5,5-dimethyl-1-(3-(5-nitro-furan-2-yl)-allyldiene-hydrazinocarbonylmethyl)-2-oxo-imidazolidin-4-yl)-1-hydroxyl-urea, EerI, ES1, Valosin-containing Protein Inhibitor II, VCP Inhibitor II, ERAD Inhibitor II, p97 Inhibitor II
About This Item
推薦產品
品質等級
化驗
≥90% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
light yellow-orange
溶解度
DMSO: 100 mg/mL
運輸包裝
ambient
儲存溫度
2-8°C
SMILES 字串
Clc1ccc(cc1)N2C(C(N(C2=O)CC(=O)NN=CC=Cc4[o]c(cc4)[N+](=O)[O-])(C)C)N(O)C(=O)Nc3ccc(cc3)Cl
InChI
1S/C27H25Cl2N7O7/c1-27(2)24(35(40)25(38)31-19-9-5-17(28)6-10-19)34(20-11-7-18(29)8-12-20)26(39)33(27)16-22(37)32-30-15-3-4-21-13-14-23(43-21)36(41)42/h3-15,24,40H,16H2,1-2H3,(H,31,38)(H,32,37)
InChI 密鑰
JTUXTPWYZXWOIB-UHFFFAOYSA-N
一般說明
包裝
警告
重構
其他說明
Wang. Q, et al. 2010. PLoS ONE5, e15479.
Cross, B.C.S., et al. 2009. J. Cell. Sci.122, 4393.
Wang, Q., et al. 2009. Proc. Natl. Acad. Sci. USA106, 2200.
Wang, Q., et al. 2008. J. Biol. Chem.283, 7445.
Fiebiger, E., et al. 2004. Mol. Biol. Cell15, 1635.
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務