推薦產品
等級
certified reference material
形狀
liquid
特點
Snap-N-Spike®/Snap-N-Shoot®
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
濃度
1.0 mg/mL in methanol with 0.1N NaOH
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
pharmaceutical (small molecule)
形式
single component solution
儲存溫度
−20°C
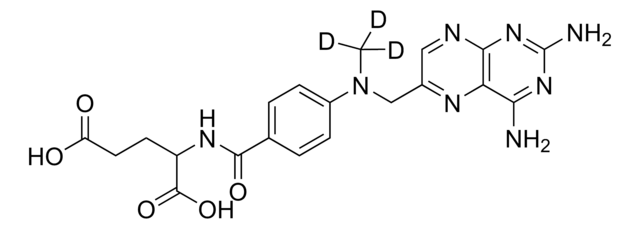
SMILES 字串
O=C(C1=CC=C(N(C)CC2=NC3=C(N=C2)N=C(N)N=C3N)C=C1)NC(C(O)=O)CCC(O)=O
InChI
1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)
InChI 密鑰
FBOZXECLQNJBKD-UHFFFAOYSA-N
一般說明
法律資訊
相關產品
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - Met. Corr. 1 - STOT SE 1
標靶器官
Eyes,Central nervous system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
51.8 °F - closed cup
閃點(°C)
11 °C - closed cup
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務