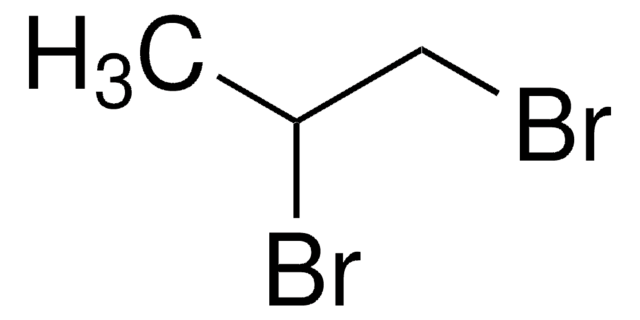
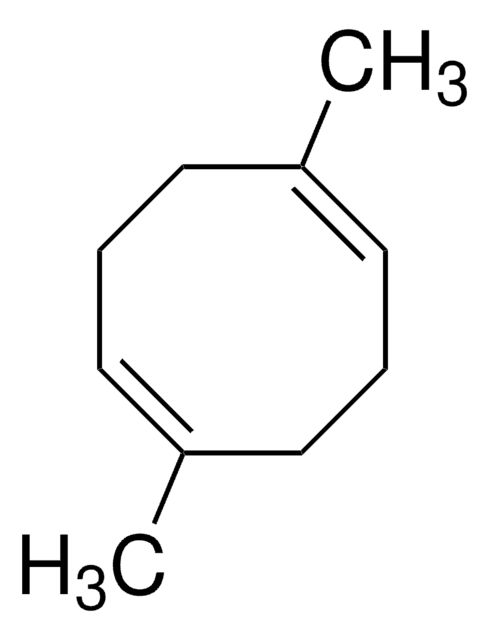
推薦產品
蒸汽壓力
25.8 mmHg ( 37.7 °C)
6.8 mmHg ( 25 °C)
化驗
99%
形狀
liquid
自燃溫度
431 °F
包含
50-150 ppm 4-tert-Butylcatechol as stabilizer
50-150 ppm 4-tert-Butylcatechol
折射率
n20/D 1.493 (lit.)
bp
149-150 °C (lit.)
mp
−69 °C (lit.)
密度
0.882 g/mL at 25 °C (lit.)
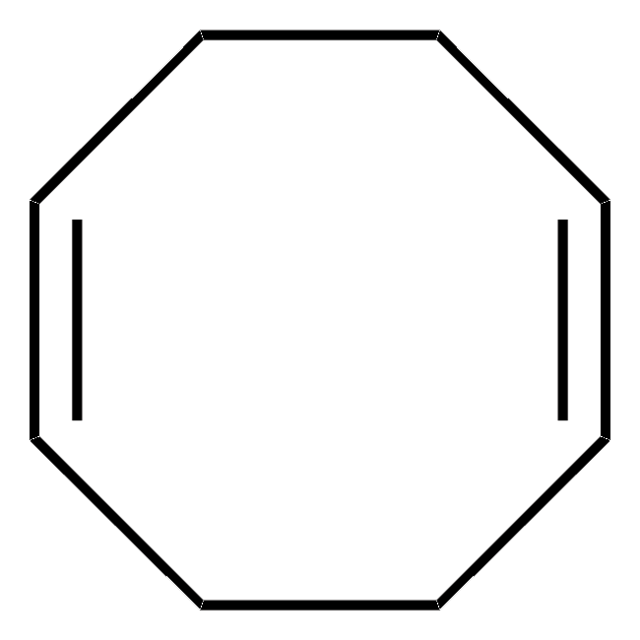
SMILES 字串
C1CC=CCCC=C1
InChI
1S/C8H12/c1-2-4-6-8-7-5-3-1/h1-2,7-8H,3-6H2/b2-1-,8-7-
InChI 密鑰
VYXHVRARDIDEHS-QGTKBVGQSA-N
尋找類似的產品? 前往 產品比較指南
應用
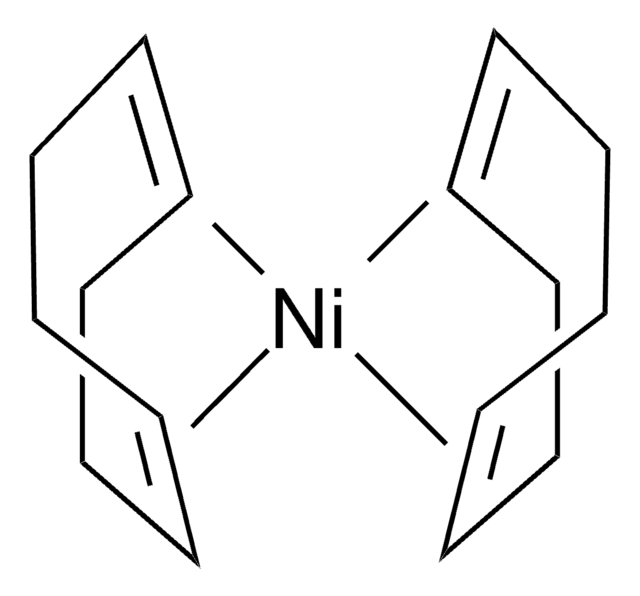
- Bis(Aluminyl)Magnesium: A Source of Nucleophilic or Radical Aluminium-Centred Reactivity.: Investigates 1,5-Cyclooctadiene′s ability to stabilize reactive intermediates in metal complexes, which is crucial for developing new pharmaceutical agents and enhancing ligand efficiency in transition metal catalysis (Griffin et al., 2024).
訊號詞
Danger
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
100.4 °F - closed cup
閃點(°C)
38 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Eleonora Cavallari et al.
The journal of physical chemistry. B, 119(31), 10035-10041 (2015-07-15)
Hyperpolarization of (13)C carboxylate signals of metabolically relevant molecules, such as acetate and pyruvate, was recently obtained by means of ParaHydrogen Induced Polarization by Side Arm Hydrogenation (PHIP-SAH). This method relies on functionalization of the carboxylic acid with an unsaturated
Adrian Tlahuext-Aca et al.
Dalton transactions (Cambridge, England : 2003), 43(42), 15997-16005 (2014-09-19)
Ni(0)-catalyzed dehydrogenation of benzylic-type imines was performed to yield asymmetrical tetra-substituted imidazoles and 2-imidazolines. This was achieved with a single operational step while maintaining good selectivity and atom economy. The catalytic system shows low to moderate tolerance for fluoro-, trifluoromethyl-
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務