推薦產品
反應適用性
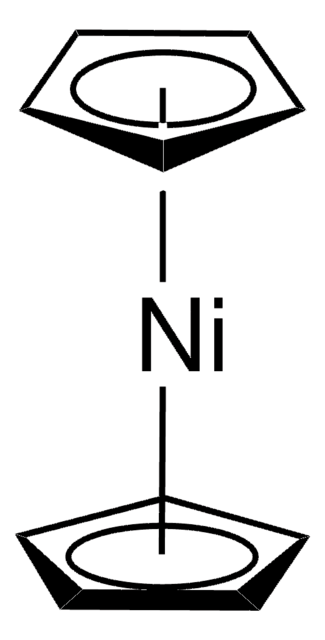
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
參數
temperature sensitive
mp
60 °C (dec.) (lit.)
儲存溫度
−20°C
SMILES 字串
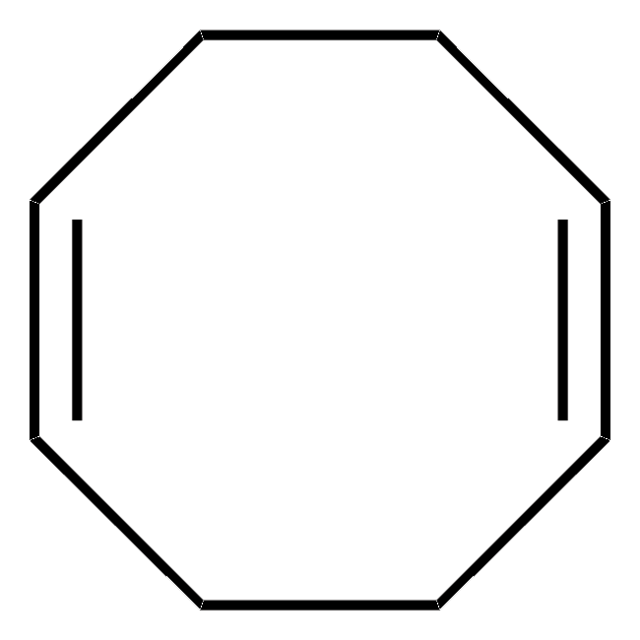
[Ni].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.Ni/c2*1-2-4-6-8-7-5-3-1;/h2*1-2,7-8H,3-6H2;/b2*2-1-,8-7-;
InChI 密鑰
JRTIUDXYIUKIIE-KZUMESAESA-N
應用
- 氧化加成反应
作为下列反应的催化剂:
- 酮与氯代芳烃的非对称α-芳基化和杂芳基化
- 交叉偶联反应
- 烯丙基醛在二氧化碳气氛中的区域选择性和立体选择性羧化/环化
- 均相炔醇的甲基羧化
- 立体选择性硼环化酮-二烯偶联
- 苯甲酰胺与内部炔烃的环加成反应
訊號詞
Danger
危險分類
Carc. 2 - Flam. Sol. 1 - Skin Sens. 1 - STOT RE 1
標靶器官
Lungs
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
文章
Csp2- and Csp-hybridized coupling reactions are established catalytic approaches. However, multi-step Csp3- and Csp2-coupling reactions of boronic acids and related derivatives are still limited by ineffective two-electron transmetalation reactions.
Csp2- and Csp-hybridized coupling reactions are established catalytic approaches. However, multi-step Csp3- and Csp2-coupling reactions of boronic acids and related derivatives are still limited by ineffective two-electron transmetalation reactions.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務