推薦產品
等級
anhydrous
品質等級
產品線
ReagentPlus®
Redi-Dri™
化驗
99%
形狀
powder or crystals (anhydrous)
品質
free-flowing
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
bp
273 °C (lit.)
mp
70-73 °C (lit.)
環保替代類別
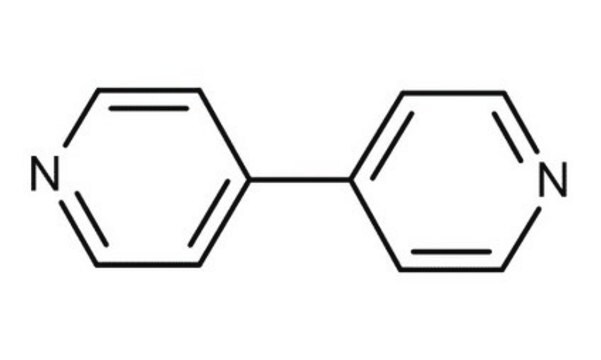
SMILES 字串
c1ccc(nc1)-c2ccccn2
InChI
1S/C10H8N2/c1-3-7-11-9(5-1)10-6-2-4-8-12-10/h1-8H
InChI 密鑰
ROFVEXUMMXZLPA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
2,2′-联吡啶是一种对称的联吡啶,通常用作与金属离子络合的中性配体。该分子是平面的,具有反式构象,并在单斜晶体系统中结晶。
我们致力于为您带来更环保的替代产品,以符合一项或多项绿色化学12项原则。该产品为增强型,提高了催化效率。点击此处以获取更多信息。
應用
2,2′-联吡啶基可用于使用 CuI/TEMPO(TEMPO = 2,2,6,6-四甲基-1-哌啶基氧基)催化剂系统通过需氧氧化从伯醇制备醛。
生化/生理作用
金属蛋白酶抑制剂,铁的高亲和力螯合剂;在10−8 M浓度下可以抑制含Fe2+的酶。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Redi-Dri is a trademark of Sigma-Aldrich Co. LLC
相關產品
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
267.8 °F - closed cup
閃點(°C)
131 °C - closed cup
客戶也查看了
The crystal structure of 2, 2'-bipyridine.
Merritt LL and Schroeder E.
Acta Crystallographica, 9(10), 801-804 (1956)
Bipyridine: the most widely used ligand. A review of molecules comprising at least two 2,2'-bipyridine units.
Kaes C, et al.
Chemical Reviews, 100(10), 3553-3590 (2000)
Jessica M Hoover et al.
Nature protocols, 7(6), 1161-1166 (2012-05-29)
This protocol describes a practical laboratory-scale method for aerobic oxidation of primary alcohols to aldehydes, using a chemoselective Cu(I)/TEMPO (TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxyl) catalyst system. The catalyst is prepared in situ from commercially available reagents, and the reactions are performed in
Kamran Shekh et al.
Comparative biochemistry and physiology. Toxicology & pharmacology : CBP, 231, 108723-108723 (2020-02-12)
Early life-stages of the endangered white sturgeon (Acipenser transmontanus) have been shown to be among the most sensitive fishes to aqueous copper (Cu) exposure. In a recent analogous study, we examined the role of whole-body Cu accumulation and Na homeostasis
Rone Aparecido De Grandis et al.
Bioorganic chemistry, 85, 455-468 (2019-02-19)
This study describes a series of newly synthesized phosphine/diimine ruthenium complexes containing the lawsone as bioligand with enhanced cytotoxicity against different cancer cells, and apoptosis induction in prostatic cancer cells DU-145. The complexes [Ru(law)(N-N)2]PF6 where N-N is 2,2'-bipyridine (1) or
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務