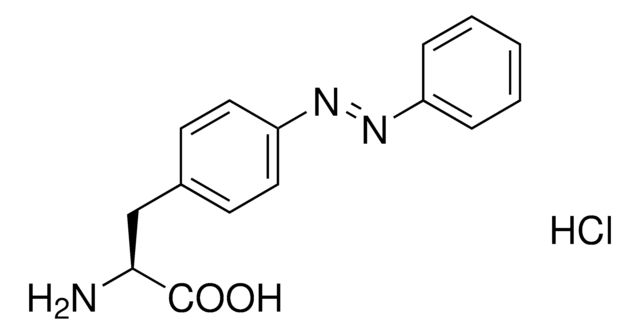
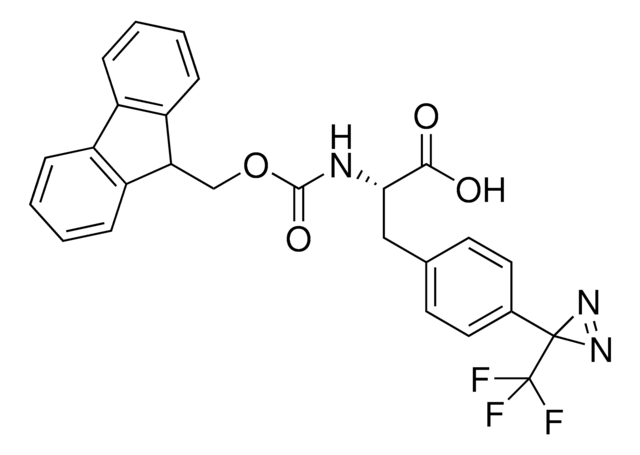
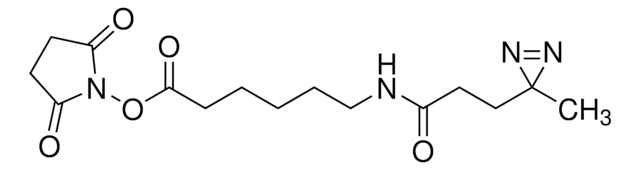
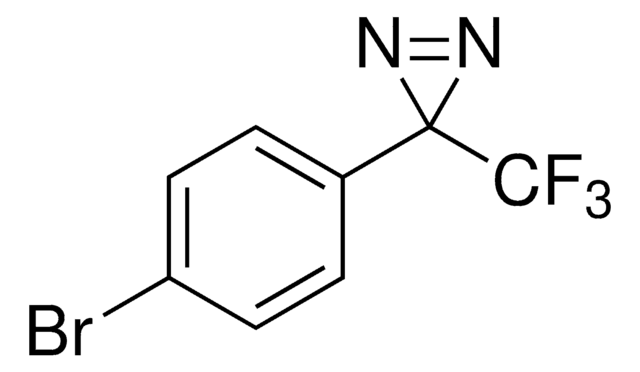
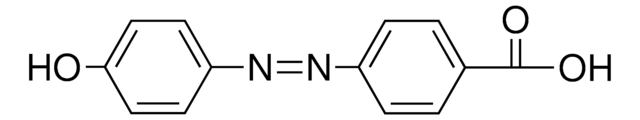
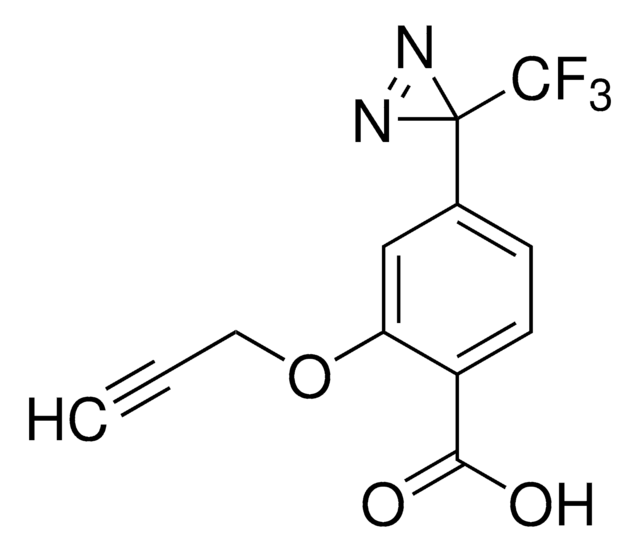
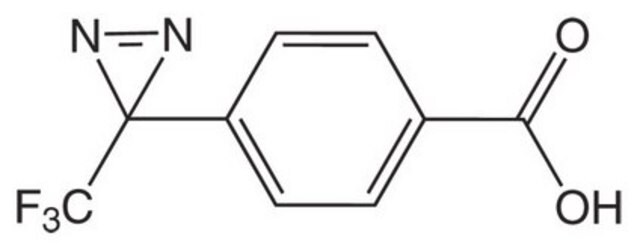
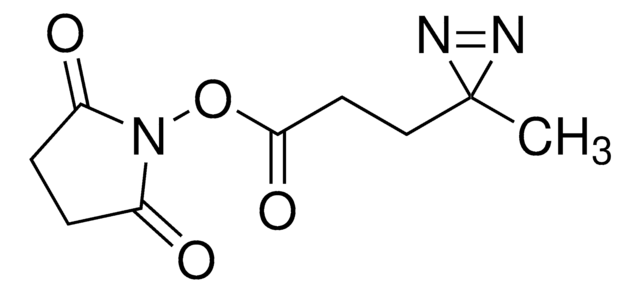
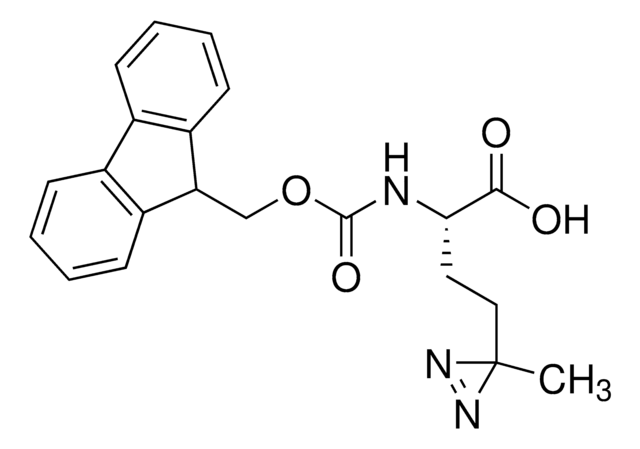
推薦產品
化驗
≥98%
形狀
powder
反應適用性
reaction type: solution phase peptide synthesis
存貨情形
available only in USA
應用
peptide synthesis
儲存溫度
−20°C
SMILES 字串
FC(F)(F)C2(N=N2)c1ccc(cc1)CC(N)C(=O)O
InChI
1S/C11H10F3N3O2/c12-11(13,14)10(16-17-10)7-3-1-6(2-4-7)5-8(15)9(18)19/h1-4,8H,5,15H2,(H,18,19)
InChI 密鑰
HRGXDARRSCSGOG-UHFFFAOYSA-N
應用
H-L-Photo-Phe-OH is a diazirine-containing phenylalanine amino acid and multifunctional
photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907294.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907294.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他說明
Genetic Incorporation of a Photo-Crosslinkable Amino Acid Reveals Novel Protein Complexes with GRB2 in Mammalian Cells
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
A genetically encoded diazirine photo-crosslinker in Escherichia coli
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
A genetically encoded diazirine photo-crosslinker in Escherichia coli
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Self-react. C
儲存類別代碼
5.2 - Organic peroxides and self-reacting hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Lei Wang et al.
Organic letters, 17(3), 616-619 (2015-01-15)
Alternative one-pot synthesis of 3-(trifluoromethyl)-3-phenyldiazirine derivatives from corresponding tosyloximes is developed. The deprotonation of intermediate diaziridine by NH2(-) is a new approach for construction of diazirine. Moreover, a novel synthesis of optically pure (trifluoromethyl)diazirinylphenylalanine derivatives was attempted involving these methods.
G Baldini et al.
Biochemistry, 27(20), 7951-7959 (1988-10-04)
The Boc-protected derivative of a photoactivatable, carbene-generating analogue of phenylalanine, L-4'-[3-(trifluoromethyl)-3H-diazirin-3-yl]phenylalanine [(Tmd)Phe], was used to acylate 5'-O-phosphorylcytidylyl(3'-5')adenosine (pCpA). A diacyl species was isolated which upon successive treatments with trifluoroacetic acid and 0.01 M HCl yielded a 1:1 mixture of 2'(3')-O-(Tmd)phenylalanyl-pCpA
Nobumasa Hino et al.
Journal of molecular biology, 406(2), 343-353 (2010-12-28)
Cell signaling pathways are essentially organized through the distribution of various types of binding domains in signaling proteins, with each domain binding to specific target molecules. Although identification of these targets is crucial for mapping the pathways, affinity-based or copurification
A genetically encoded diazirine photocrosslinker in Escherichia coli.
Eric M Tippmann et al.
Chembiochem : a European journal of chemical biology, 8(18), 2210-2214 (2007-11-15)
Lei Wang et al.
Bioscience, biotechnology, and biochemistry, 78(7), 1129-1134 (2014-09-18)
In this paper we report here a hydrogen/deuterium exchange (H/D exchange) of cross-linkable α-amino acid derivatives with deuterated trifluoromethanesulfonic acid (TfOD). H/D exchange with TfOD was easily applied to o-catechol containing phenylalanine (DOPA) within an hour. A partial H/D exchange
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務