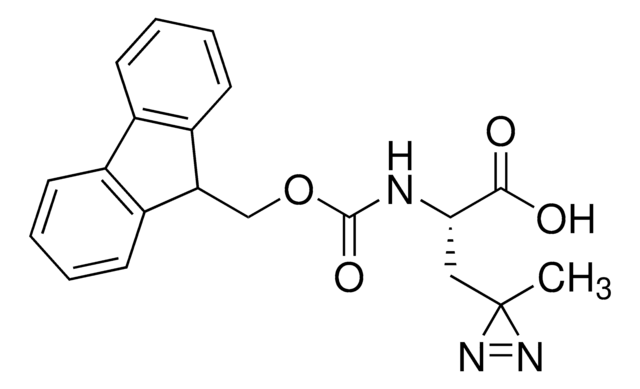
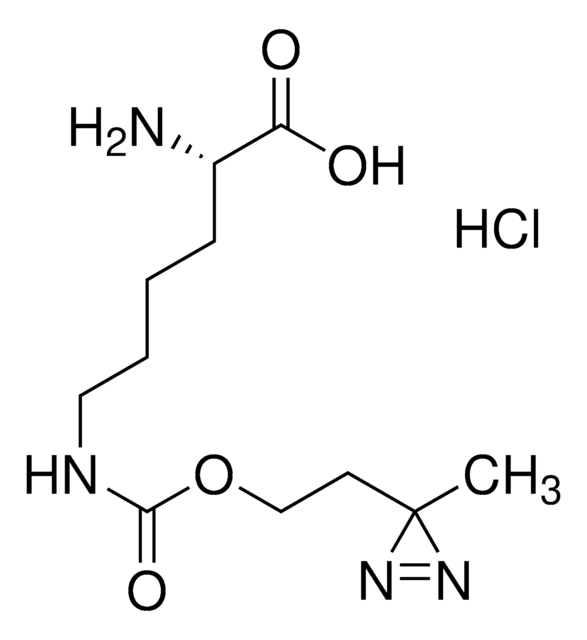
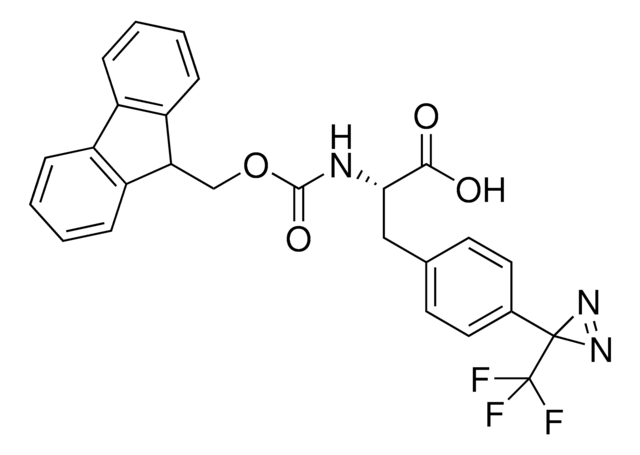
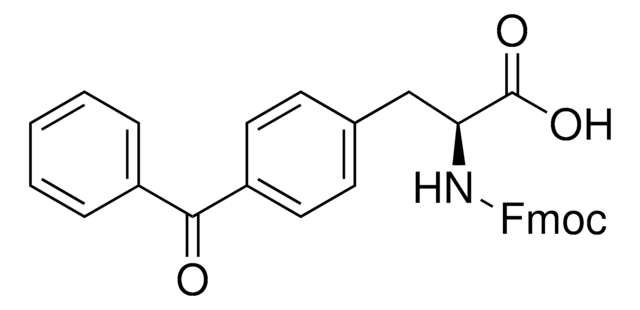
907278
H-L-Photo-leucine HCl
≥98%
同義詞:
(S)-2-Amino-3-(3-methyl-3H-diazirin-3-yl)propanoic acid hydrochloride, (S)-2-Amino-3-(3H-diazirin-3-yl)butanoic acid hydrochloride, Diazirine amino acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
登入查看組織和合約定價
全部照片(1)
About This Item
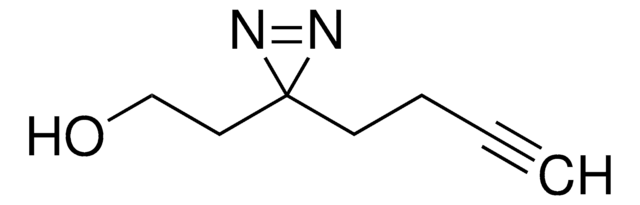
經驗公式(希爾表示法):
C5H10ClN3O2
分子量::
179.60
分類程式碼代碼:
12352209
推薦產品
化驗
≥98%
形狀
solid
反應適用性
reaction type: solution phase peptide synthesis
存貨情形
available only in USA
應用
peptide synthesis
儲存溫度
−20°C
應用
H-L-Photo-leucine HCl is a diazirine-containing leucine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907391.
其他說明
Development, optimization, and structural characterization of an efficient peptide-based photoaffinity cross-linking reaction
for generation of homogeneous conjugates from wild-type antibodies
Mechanistic studies of a small-molecule modulator of SMN2 splicing
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Direct Interaction between an Allosteric Agonist Pepducin and the Chemokine Receptor CXCR4
Photo-leucine and photo-methionine allow identification of protein-?protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
for generation of homogeneous conjugates from wild-type antibodies
Mechanistic studies of a small-molecule modulator of SMN2 splicing
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Direct Interaction between an Allosteric Agonist Pepducin and the Chemokine Receptor CXCR4
Photo-leucine and photo-methionine allow identification of protein-?protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Self-react. C
儲存類別代碼
5.2 - Organic peroxides and self-reacting hazardous materials
水污染物質分類(WGK)
WGK 3
客戶也查看了
Yuan Gao et al.
Chemical communications (Cambridge, England), 48(67), 8404-8406 (2012-07-18)
Here we report the first example of the use of supramolecular hydrogels to discover the protein targets of aggregates of small molecules.
Jingxin Wang et al.
Proceedings of the National Academy of Sciences of the United States of America, 115(20), E4604-E4612 (2018-05-02)
RG-7916 is a first-in-class drug candidate for the treatment of spinal muscular atrophy (SMA) that functions by modulating pre-mRNA splicing of the SMN2 gene, resulting in a 2.5-fold increase in survival of motor neuron (SMN) protein level, a key protein
Nicholas Vance et al.
Bioconjugate chemistry, 30(1), 148-160 (2018-12-20)
Site-specific conjugation of small molecules to antibodies represents an attractive goal for the development of more homogeneous targeted therapies and diagnostics. Most site-specific conjugation strategies require modification or removal of antibody glycans or interchain disulfide bonds or engineering of an
Balakrishnan S Moorthy et al.
Journal of visualized experiments : JoVE, (98), 52503-52503 (2015-05-06)
Amide hydrogen/deuterium exchange (ssHDX-MS) and side-chain photolytic labeling (ssPL-MS) followed by mass spectrometric analysis can be valuable for characterizing lyophilized formulations of protein therapeutics. Labeling followed by suitable proteolytic digestion allows the protein structure and interactions to be mapped with
Lavanya K Iyer et al.
Molecular pharmaceutics, 10(12), 4629-4639 (2013-10-16)
Local side-chain interactions in lyophilized protein formulations were mapped using solid-state photolytic labeling-mass spectrometry (ssPL-MS). Photoactive amino acid analogues (PAAs) were used as probes and either added to the lyophilized matrix or incorporated within the amino acid sequence of a
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務