907367
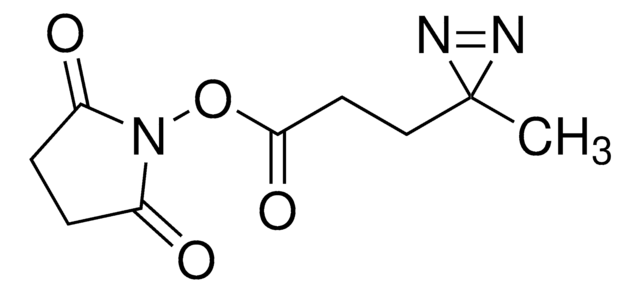
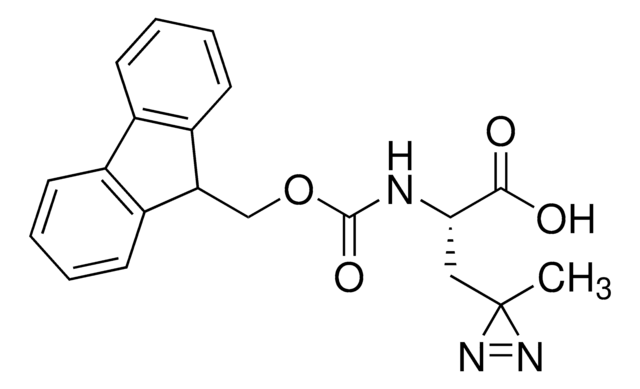
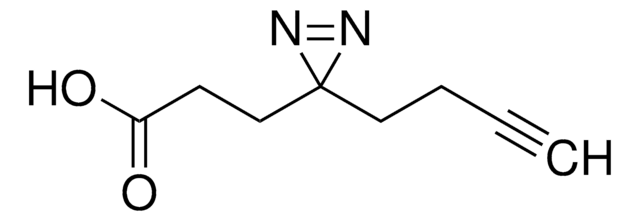
Fmoc-L-photo-methionine
≥95%, for peptide synthesis
同義詞:
(S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-4-(3-methyl-3H-diazirin-3-yl)butanoic acid, (S)-2-(Fmoc-amino)-4-(3H-diazirin-3-yl)pentanoic acid, Diazirine amino acid, Photo-Met, Photo-crosslinking amino acid, Photoprobe building block
登入查看組織和合約定價
全部照片(1)
About This Item
推薦產品
產品名稱
Fmoc-L-photo-methionine, ≥95%
化驗
≥95%
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
應用
peptide synthesis
官能基
Fmoc
儲存溫度
−20°C
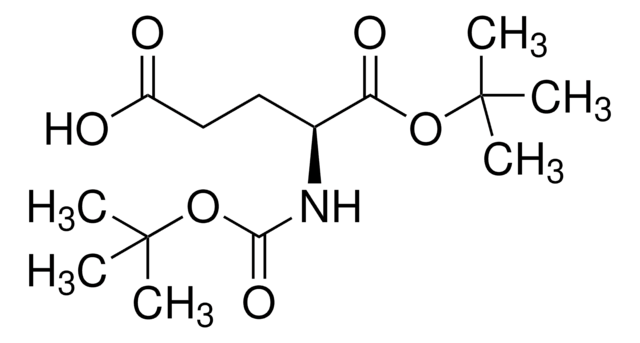
SMILES 字串
N([C@@H](CCC4(N=N4)C)C(=O)O)C(=O)OCC1c2c(cccc2)c3c1cccc3
InChI 密鑰
QKMQUEIDJLPYHS-SFHVURJKSA-N
應用
Fmoc-L-photo-methionine is a diazirine-containing, Fmoc-protected methionine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907375.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他說明
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers ′
Proteome profiling reveals potential cellular targets of staurosporine using a clickable cell-permeable probe
Covalent Capture of Phospho-Dependent Protein Oligomerization by Site-Specific Incorporation of a Diazirine Photo-Cross-Linker
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Proteome profiling reveals potential cellular targets of staurosporine using a clickable cell-permeable probe
Covalent Capture of Phospho-Dependent Protein Oligomerization by Site-Specific Incorporation of a Diazirine Photo-Cross-Linker
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Covalent capture of phospho-dependent protein oligomerization by site-specific incorporation of a diazirine photo-cross-linker.
Miquel Vila-Perelló et al.
Journal of the American Chemical Society, 129(26), 8068-8069 (2007-06-15)
Haibin Shi et al.
Chemical communications (Cambridge, England), 47(40), 11306-11308 (2011-09-17)
A clickable, affinity-based probe (AfBP), which was modified from staurosporine (a natural product kinase inhibitor), has been synthesized and used in situ for activity-based proteome profiling of potential cellular targets of staurosporine in HepG2 cancer cells.
Developing diazirine-based chemical probes to identify histone modification 'readers' and 'erasers'.
Tangpo Yang et al.
Chemical science, 6(2), 1011-1017 (2015-02-01)
Post translational modifications (PTMs, e.g., phosphorylation, acetylation and methylation) of histone play important roles in regulating many fundamental cellular processes such as gene transcription, DNA replication and damage repair. While 'writer' and 'eraser' enzymes modify histones by catalyzing the addition
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務