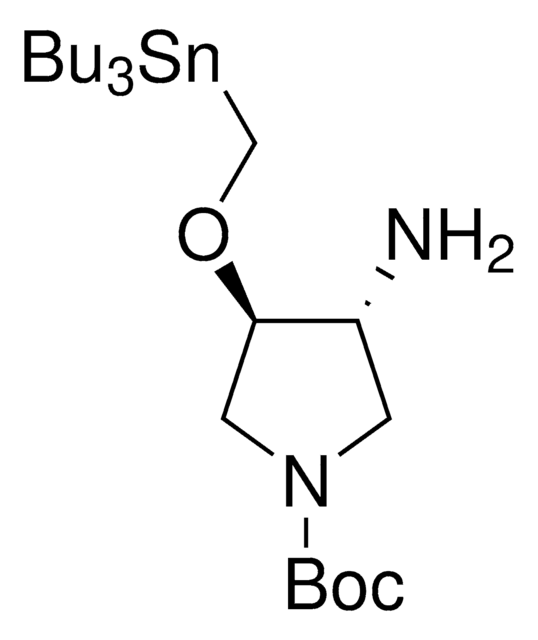
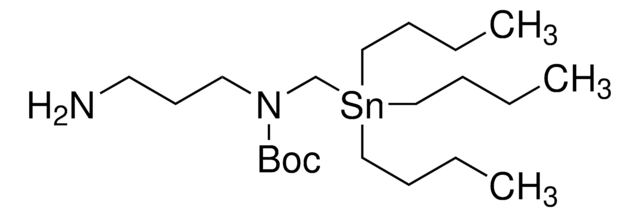
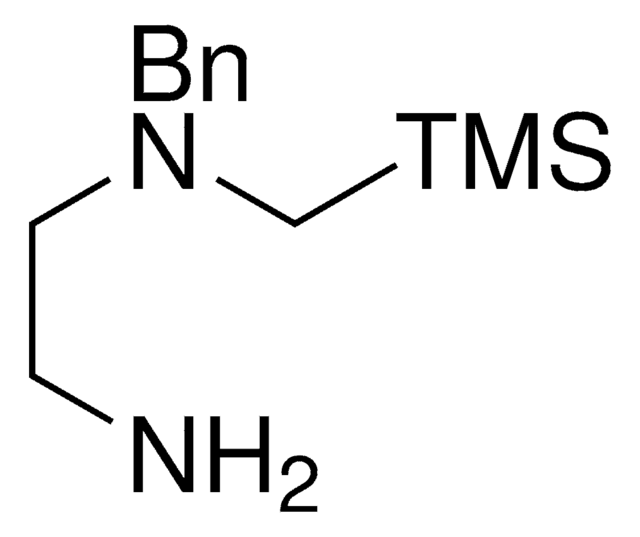
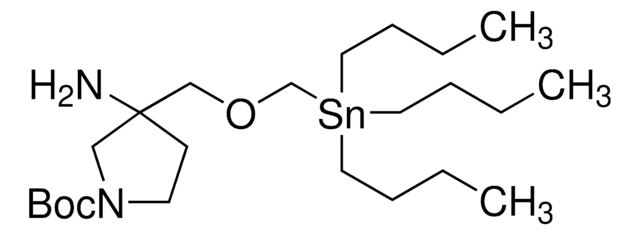
About This Item
推薦產品
形狀
liquid
品質等級
折射率
n/D 1.4860
密度
1.108 g/mL
官能基
amine
carbamate
儲存溫度
−20°C
SMILES 字串
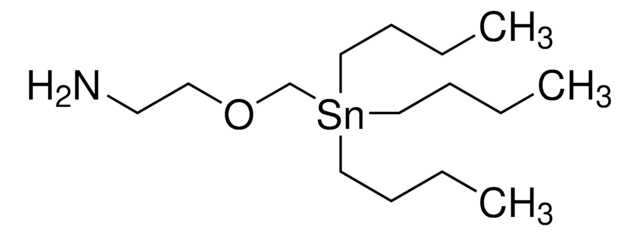
CCCC[Sn](CCCC)(CN(C(OC(C)(C)C)=O)CCN)CCCC
InChI
1S/C8H17N2O2.3C4H9.Sn/c1-8(2,3)12-7(11)10(4)6-5-9;3*1-3-4-2;/h4-6,9H2,1-3H3;3*1,3-4H2,2H3;
InChI 密鑰
LGYNPAYNCSWDHV-UHFFFAOYSA-N
應用
Automate your N-heterocycle formation with Synple Automated Synthesis Platform (SYNPLE-SC002)
其他說明
Professor product portal: Jeffrey Bode Research Group
SnAP Reagents for the Synthesis of Piperazines and Morpholines
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes
SnAP Reagents for a Cross-Coupling Approach to the One-Step Synthesis of Saturated N-Heterocycles
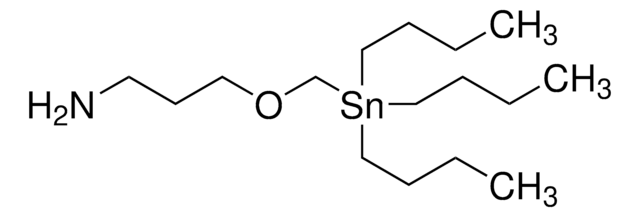
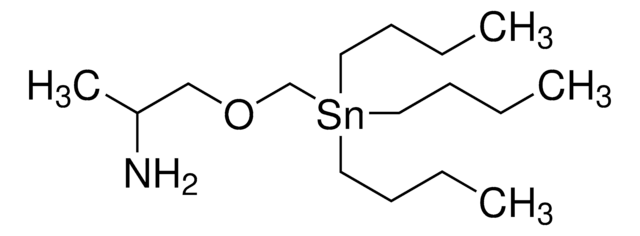
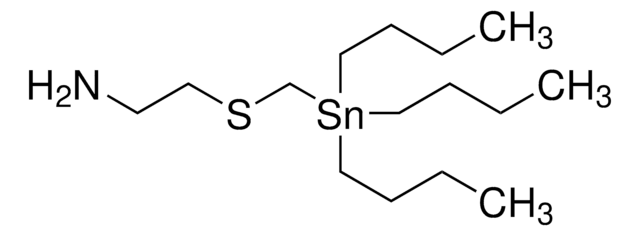
相關產品
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
條款
Saturated N-heterocyclic building blocks or SnAP Reagents are of growing importance for the convenient synthesis of medium-ring saturated N-heterocycles, including bicyclic and spirocyclic structures. SnAP reagents are stable and readily available and can be coupled with widely available aromatic, heteroaromatic, aliphatic, and glyoxylic aldehydes.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務