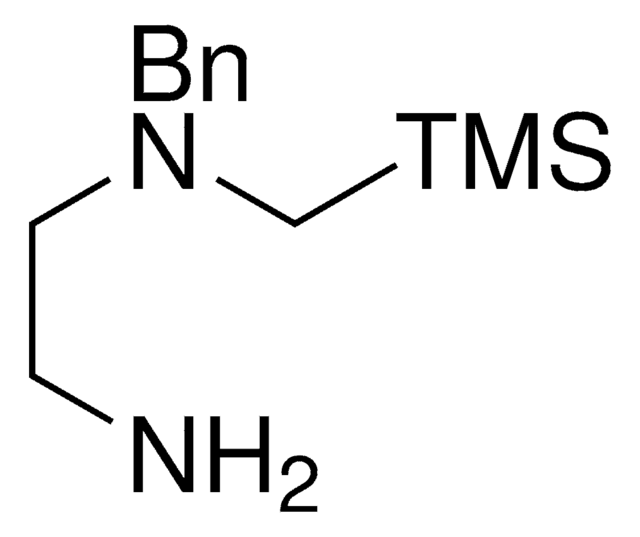
推薦產品
化驗
≥95%
形狀
liquid
折射率
n/D 1.4811
密度
0.90746 g/mL
官能基
amine
thioether
儲存溫度
2-8°C
SMILES 字串
NCCSC[Si](C)(C)C
應用
When used with aliphatic or aromatic aldehydes, this silicon amine protocol (SLAP) reagent enables the photomediated synthesis of thiomorpholines and thiazepanes. For photocatalytic cross-coupling with this SLAP Reagent, Ir[(ppy)2dtbbpy]PF6 ((747769) is used with a recommended starting combination of Lewis acids Bi(OTf)3 (633305) and Cu(OTf)2 (283673) prior to subsequent substrate-specific optimization. This product was introduced in collaboration with the Bode Research Group and provides a tin-free alternative to SnAP (tin amine protocol) reagents and is well-suited for scale-up reactions.
其他說明
- Technology Spotlight: SLAP Reagents for Piperazine Synthesis
- Silicon Amine Reagents for the Photocatalytic Synthesis of Piperazines from Aldehydes and Ketones
- Lewis Acid Induced Toggle from Ir(II) to Ir(IV) Pathways in Photocatalytic Reactions: Synthesis of Thiomorpholines and Thiazepanes from Aldehydes and SLAP Reagents.
- Continuous Flow Synthesis of Morpholines and Oxazepanes with Silicon Amine Protocol (SLAP) Reagents and Lewis Acid Facilitated Photoredox Catalysis
相關產品
產品號碼
描述
訂價
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
174.0 °F
閃點(°C)
78.89 °C
Moritz K Jackl et al.
Organic letters, 19(17), 4696-4699 (2017-08-17)
Photocatalytic coupling of aldehydes and silicon amine protocol (SLAP) reagents enables the simple, scalable synthesis of substituted morpholines, oxazepanes, thiomorpholines, and thiazepanes under continuous flow conditions. Key to the success of this process is the combination of an inexpensive organic
Sheng-Ying Hsieh et al.
Organic letters, 18(9), 2098-2101 (2016-04-22)
Silicon amine protocol (SLAP) reagents for photocatalytic cross-coupling with aldehydes and ketones to form N-unprotected piperazines have been developed. This blue light promoted process tolerates a wide range of heteroaromatic, aromatic, and aliphatic aldehydes and structurally and stereochemically complex SLAP
Sheng-Ying Hsieh et al.
ACS central science, 3(1), 66-72 (2017-02-06)
Redox neutral photocatalytic transformations often require careful pairing of the substrates and photoredox catalysts in order to achieve a catalytic cycle. This can limit the range of viable transformations, as we recently observed in attempting to extend the scope of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務