推薦產品
形狀
liquid
品質等級
反應適用性
reaction type: click chemistry
reagent type: linker
官能基
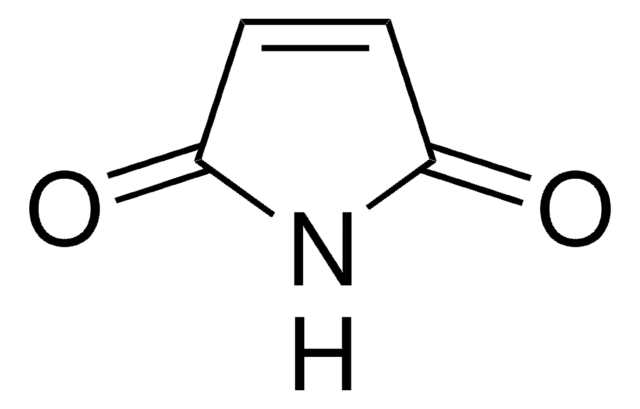
maleimide
儲存溫度
−20°C
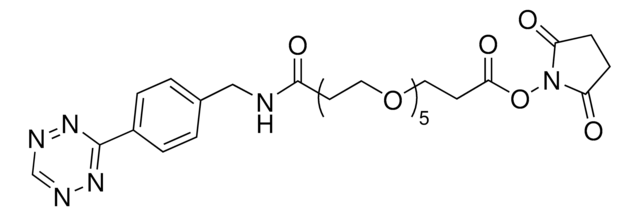
SMILES 字串
O=C(NCCCOCCOCCOCCCNC(CCN1C(C=CC1=O)=O)=O)OC2CCC/C=C/CC2
InChI
1S/C26H41N3O8/c30-23(12-15-29-24(31)10-11-25(29)32)27-13-6-16-34-18-20-36-21-19-35-17-7-14-28-26(33)37-22-8-4-2-1-3-5-9-22/h1-2,10-11,22H,3-9,12-21H2,(H,27,30)(H,28,33)/b2-1+
InChI 密鑰
BJZRPWAVCDJCTC-OWOJBTEDSA-N
生化/生理作用
Maleimide (thiol reactive) functionalized trans-cyclooctene derivative for incorporation of the cyclooctene moiety into thiol containing compounds or biomolecules. Trans-cyclooctenes are useful in strain-promoted copper-free click chemistry cycloaddition reactions with 1; 2; 4; 5-tetrazines. This cyclooctene will react with tetrazine functionalized compounds or biomolecules without the need for a catalyst to result in a stable covalent linkage. The 4+2 inverse electron demand Diels-Alder cycloaddition between trans-cyclooctene and tetrazines is the fastest biologically compatible ligation technology reported and has had many applications in biological labeling and imaging. The PEG spacer allows for increased water solubility; less aggregation and an increased distance between the thiol to be modified and the reactive alkene.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
René Platzer et al.
Nature communications, 11(1), 4993-4993 (2020-10-07)
Determining nanoscale protein distribution via Photoactivated Localization Microscopy (PALM) mandates precise knowledge of the applied fluorophore's blinking properties to counteract overcounting artifacts that distort the resulting biomolecular distributions. Here, we present a readily applicable methodology to determine, optimize and quantitatively
Mark R Karver et al.
Bioconjugate chemistry, 22(11), 2263-2270 (2011-09-29)
1,2,4,5-Tetrazines have been established as effective dienes for inverse electron demand [4 + 2] Diels-Alder cycloaddition reactions with strained alkenes for over 50 years. Recently, this reaction pair combination has been applied to bioorthogonal labeling and cell detection applications; however
Neal K Devaraj et al.
Bioconjugate chemistry, 19(12), 2297-2299 (2008-12-05)
Bioorthogonal tetrazine cycloadditions have been applied to live cell labeling. Tetrazines react irreversibly with the strained dienophile norbornene forming dihydropyrazine products and dinitrogen. The reaction is high yielding, selective, and fast in aqueous media. Her2/neu receptors on live human breast
Melissa L Blackman et al.
Journal of the American Chemical Society, 130(41), 13518-13519 (2008-09-19)
Described is a bioorthogonal reaction that proceeds with unusually fast reaction rates without need for catalysis: the cycloaddition of s-tetrazine and trans-cyclooctene derivatives. The reactions tolerate a broad range of functionality and proceed in high yield in organic solvents, water
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 790443-25MG | 4061833231982 |
| 790443-5MG | 4061833231999 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務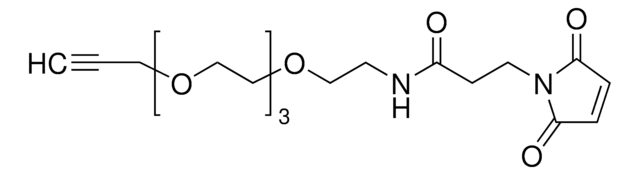

![9-氧杂二环[6.1.0]壬-4-烯 95%](/deepweb/assets/sigmaaldrich/product/structures/328/338/c44d0a8d-81ab-4a17-81bb-aebb26a006e7/640/c44d0a8d-81ab-4a17-81bb-aebb26a006e7.png)

![(1R,8S,9s)-双环[6.1.0]壬-4-炔-9-基甲基 N-琥珀酰亚胺碳酸酯 for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)