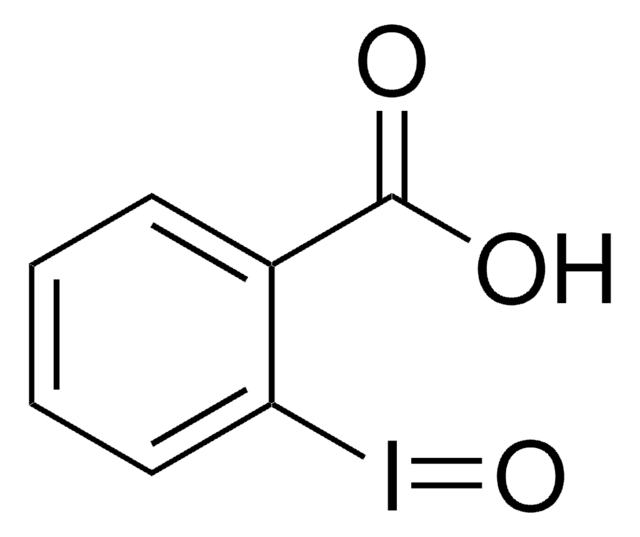
About This Item
推薦產品
品質等級
化驗
95%
形狀
powder
反應適用性
reaction type: C-C Bond Formation
mp
75-79 °C
官能基
fluoro
iodo
儲存溫度
2-8°C
SMILES 字串
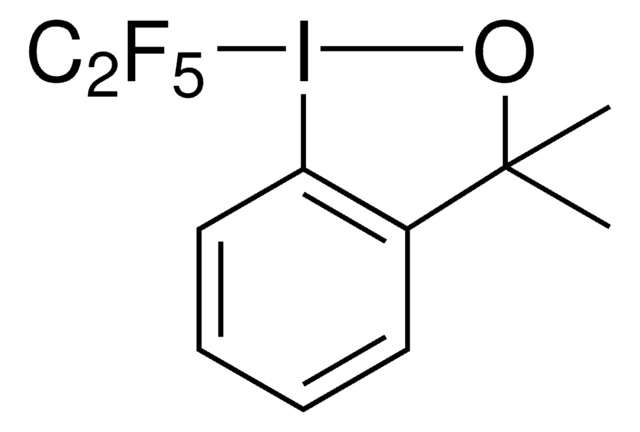
CC1(C)O[I](c2ccccc12)C(F)(F)F
InChI
1S/C10H10F3IO/c1-9(2)7-5-3-4-6-8(7)14(15-9)10(11,12)13/h3-6H,1-2H3
InChI 密鑰
HVAPLSNCVYXFDQ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
多种化合物的三氟甲基化包括:
- 仲和伯芳基和烷基膦
- 酚类
- 经过SPPS和亲电子S-三氟甲基化的含有半胱氨酸的肽
- 芳烃和N-杂环
- Arozoles的亲电N-三氟甲基化
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
文章
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 696641-250MG | 4061832785608 |
| 696641-5G | 4061833338353 |
| 696641-1G | 4061832785554 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
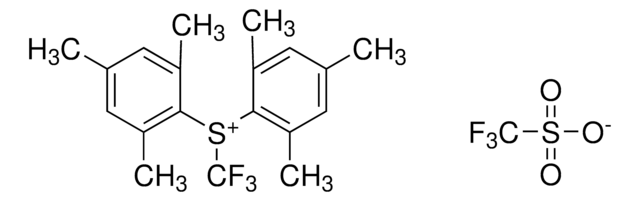
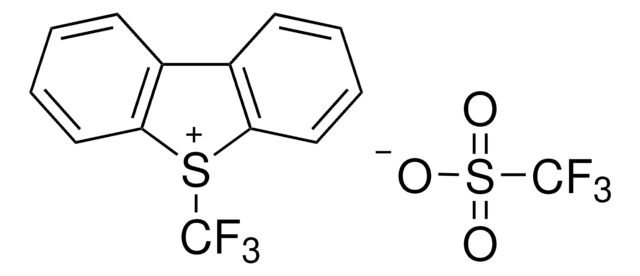
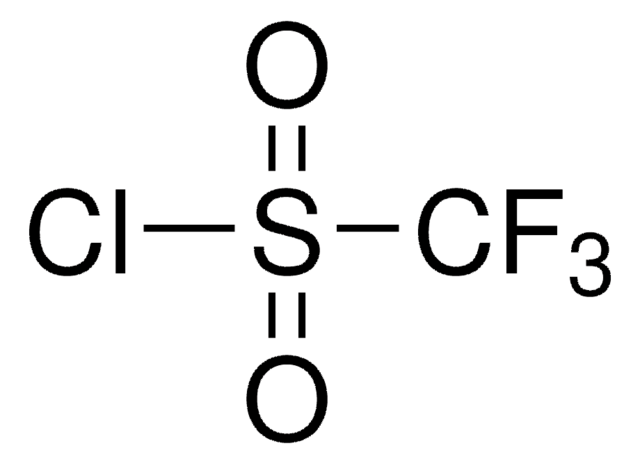

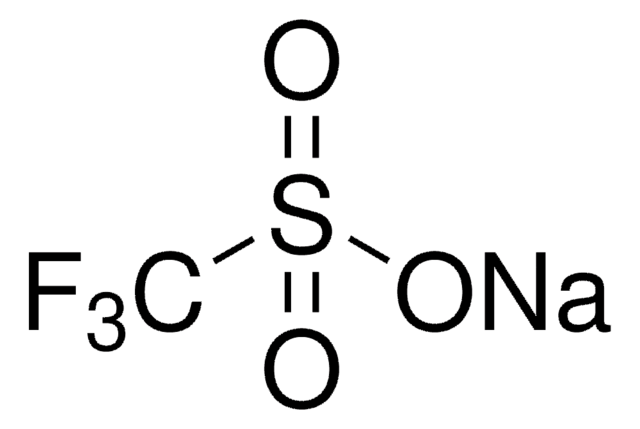


![1-[(Triisopropylsilyl)ethynyl]-1,2-benziodoxol-3(1H)-one ≥98.0% (AT)](/deepweb/assets/sigmaaldrich/product/structures/306/080/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d/640/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d.png)