全部照片(1)
About This Item
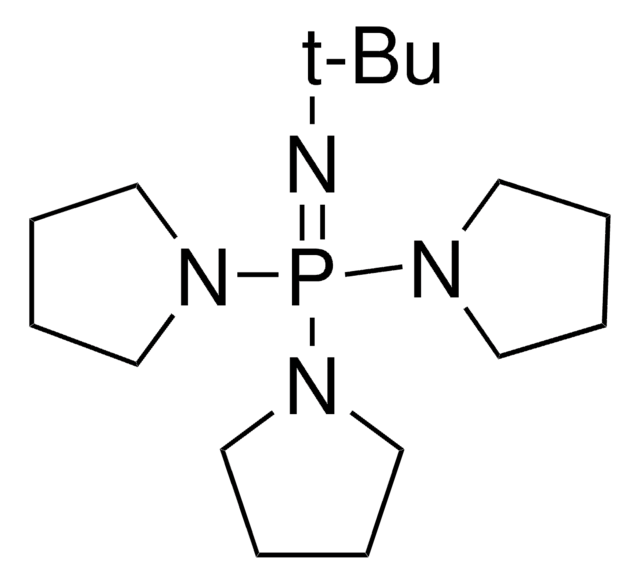
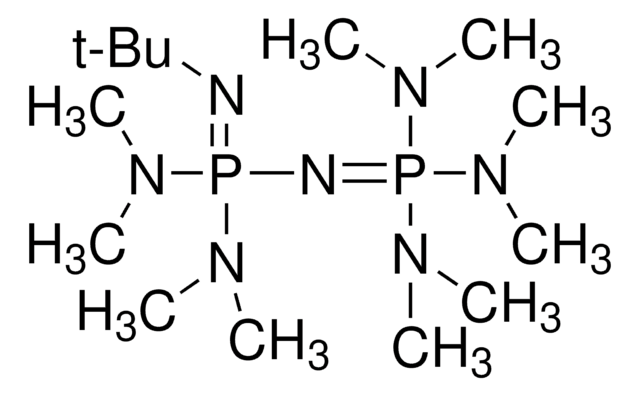
經驗公式(希爾表示法):
C18H39N4P
CAS號碼:
分子量::
342.50
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
97%
折射率
n20/D 1.5020 (lit.)
密度
0.964 g/mL at 25 °C (lit.)
SMILES 字串
CC(C)CN1CCN2CCN(CC(C)C)P1N(CC2)CC(C)C
InChI
1S/C18H39N4P/c1-16(2)13-20-10-7-19-8-11-21(14-17(3)4)23(20)22(12-9-19)15-18(5)6/h16-18H,7-15H2,1-6H3
InChI 密鑰
WFHPXSHLCFHEIA-UHFFFAOYSA-N
應用
与 Pd2(dba)3(货号 328774)形成高效催化剂,用于一锅法合成反式-4-N,N-二芳氨基二苯乙烯和 N,N-二芳氨基苯乙烯。作为催化剂用于杂芳族氨基甲酸酯的甲醇裂解。
作为配体用于腈与芳基溴化物和氯化物的 α-芳基化反应,以及用于芳基氯化物的 Stille 交叉偶联反应。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
154.9 °F - closed cup
閃點(°C)
68.3 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
客戶也查看了
Weiping Su et al.
Organic letters, 6(9), 1421-1424 (2004-04-23)
[reaction: see text] The Pd(2)(dba)(3)/P(i-BuNCH(2)CH(2))(3)N (1d) catalyst system is highly effective for the Stille cross-coupling of aryl chlorides with organotin compounds. This method represents only the second general method for the coupling of aryl chlorides. Other proazaphosphatranes possessing benzyl substituents
Tetrahedron Letters, 47, 5645-5645 (2006)
A general method for the direct alpha-arylation of nitriles with aryl chlorides.
Jingsong You et al.
Angewandte Chemie (International ed. in English), 42(41), 5051-5053 (2003-11-05)
P(i-BuNCH2CH2)3N: an efficient ligand for the direct alpha-arylation of nitriles with aryl bromides.
Jingsong You et al.
The Journal of organic chemistry, 68(21), 8003-8007 (2003-10-11)
A new catalyst system for the synthesis of alpha-aryl-substituted nitriles is reported. The bicyclic triaminophosphine P(i-BuNCH(2)CH(2))(3)N (1b) serves as an efficient and versatile ligand for the palladium-catalyzed direct alpha-arylation of nitriles with aryl bromides. Using ligand 1b, ethyl cyanoacetate and
Tetrahedron, 61, 9775-9775 (2005)
文章
Proazaphosphatranes: Verkade’s Superbases
An article on Proazaphosphatranes: Verkade’s Superbases.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![2,8,9-三异丙基-2,5,8,9-四硫唑嘌呤-1-磷杂双环[3,3,3]十一烷](/deepweb/assets/sigmaaldrich/product/structures/387/021/edaffe12-6e4b-4305-9030-749551ac828a/640/edaffe12-6e4b-4305-9030-749551ac828a.png)
![1,5,7-三氮杂双环 [4.4.0] 癸烯-5-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
![[Pd2(dba)3] x dba Umicore](/deepweb/assets/sigmaaldrich/product/structures/150/531/11e74f1a-c256-4d30-b43d-8c299f1034b1/640/11e74f1a-c256-4d30-b43d-8c299f1034b1.png)