推薦產品
產品名稱
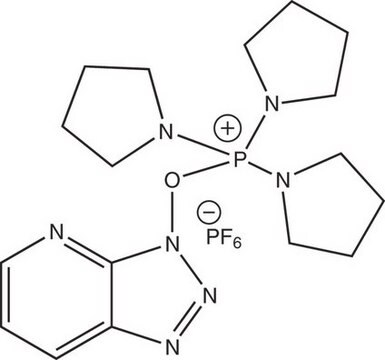
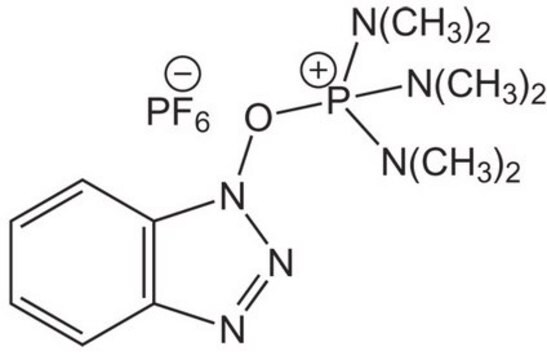
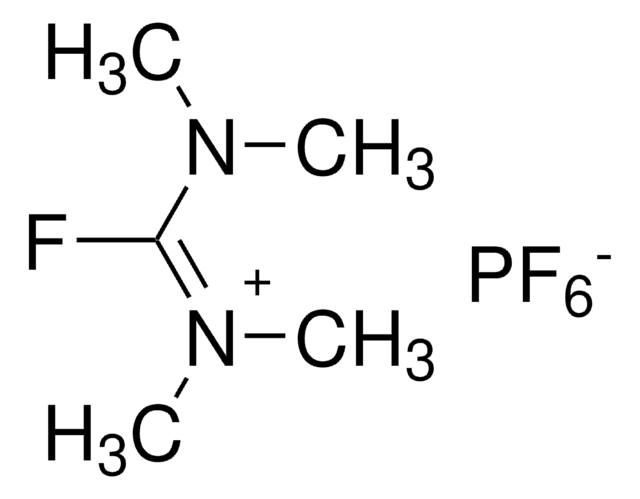
六氟磷酸(7-氮杂苯并三唑-1-氧基)三吡咯烷磷, 96%
品質等級
化驗
96%
反應適用性
reaction type: Coupling Reactions
mp
163-168 °C (lit.)
應用
peptide synthesis
儲存溫度
−20°C
SMILES 字串
F[P-](F)(F)(F)(F)F.C1CCN(C1)[P+](On2nnc3cccnc23)(N4CCCC4)N5CCCC5
InChI
1S/C17H27N7OP.F6P/c1-2-11-21(10-1)26(22-12-3-4-13-22,23-14-5-6-15-23)25-24-17-16(19-20-24)8-7-9-18-17;1-7(2,3,4,5)6/h7-9H,1-6,10-15H2;/q+1;-1
InChI 密鑰
CBZAHNDHLWAZQC-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
應用
作为合成以下物质的试剂:
通过点击化学官能团化的环 RGC 五肽
荧光葡萄糖生物探针
ReactIR™ 流式细胞
用于以下反应的试剂:
二硫键工程
杂芳基醚的合成
Grb2-SH2 结构域非磷酸化环肽拮抗剂的构象限制优化
通过点击化学官能团化的环 RGC 五肽
荧光葡萄糖生物探针
ReactIR™ 流式细胞
用于以下反应的试剂:
二硫键工程
杂芳基醚的合成
Grb2-SH2 结构域非磷酸化环肽拮抗剂的构象限制优化
法律資訊
经 PE Biosystems 授权销售
ReactIR is a trademark of Mettler-Toledo, Inc.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Zhaoguan Wu et al.
Scientific reports, 7, 46206-46206 (2017-04-08)
O-Acetylation of sialic acid in protein N-glycans is an important modification and can occur at either 4-, 7-, 8- or 9-position in various combinations. This modification is usually labile under alkaline reaction conditions. Consequently, a permethylation-based analytical method, which has
Si Liu et al.
Journal of proteomics, 181, 225-237 (2018-04-27)
Colorectal cancer (CRC) has become one of the most common cancers worldwide and the fifth most prevalent cancer in China with an upward trend in incidence rates. Altered glycosylation significantly affects the structural and functional changes in immunoglobulin G (IgG)
Yike Wu et al.
Analytical and bioanalytical chemistry, 409(16), 4027-4036 (2017-04-19)
A rapid and sensitive N-glycan profiling strategy for MALDI-MS incorporating the use of deglycosylation with microwave assistance and the co-derivatization of glycosylamine labeling with tris(2,4,6-trimethoxyphenyl)phosphonium acetic acid N-hydroxysuccinimide ester (TMPP-Ac-OSu) and methylamidation has been developed in this work. Notably, highly
Chang Wang et al.
Analytica chimica acta, 1002, 50-61 (2018-01-08)
Quantitative analysis of glycans is an emerging field in glycomic research. Herein we present a rapid and effective dual-labeling strategy, in the combination of isotopic derivatization of N-glycosylamine-based glycans by d0/d5-benzoyl chloride and methylamidation of sialic acids, to relatively quantify
Tarshona Stevens et al.
International journal of medicinal chemistry, 2012, 730239-730239 (2012-01-01)
The purpose of this study is to understand the interactions of some antibacterial cationic amphipathic cyclooctapeptides with calcium(II) and their secondary structural preferences. The thermodynamic parameters associated with calcium(II) interactions, between the antibacterial active cyclooctapeptides (COP 1-6) and those that
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 535303-1G | 4061826732977 |
| 535303-5G | 4061832565118 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務