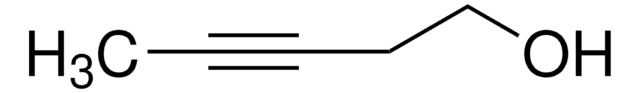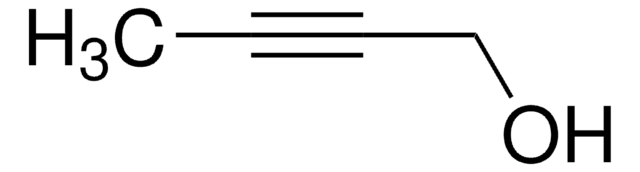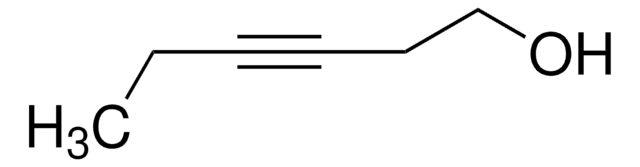推薦產品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.452 (lit.)
bp
84-85 °C/57 mmHg (lit.)
密度
0.909 g/mL at 25 °C (lit.)
SMILES 字串
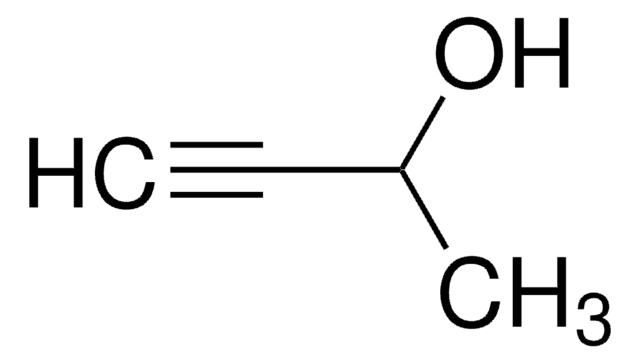
CCC#CCO
InChI
1S/C5H8O/c1-2-3-4-5-6/h6H,2,5H2,1H3
InChI 密鑰
WLPYSOCRPHTIDZ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
2-Pentyn-1-ol was employed as starting reagent for the synthesis of (-)-muricatacin. It was also used in the preparation of (2Z)-3-tributylstannyl-2-penten-1-ol.
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
136.4 °F - closed cup
閃點(°C)
58 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
A Wada et al.
Chemical & pharmaceutical bulletin, 48(9), 1391-1394 (2000-09-19)
Palladium catalyzed cross coupling reactions of a vinyl triflate intermediate and various alkenyl stannanes afforded trisubstituted Z-olefins stereoselectively in high yields. These olefins were then converted to the corresponding 9Z-retinoic acids via Horner-Emmons reaction and subsequent basic hydrolysis in excellent
H Makabe et al.
Bioscience, biotechnology, and biochemistry, 57(6), 1028-1029 (1993-06-01)
The synthesis of (-)-muricatacin starting from 1-bromododecane and 2-pentyn-1-ol is described. 2-Pentadecyn-1-ol (4), which was prepared from 1-bromododecane (2) and 2-pentyn-1-ol (3), was converted to epoxy alcohol 6 through a two-step reaction sequence, 6 being successively submitted to tosylation, iodination
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 335312-5G | 4061838090362 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務