推薦產品
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.418 (lit.)
bp
73-75 °C (lit.)
mp
−61 °C (lit.)
密度
0.913 g/mL at 25 °C (lit.)
官能基
alkyl halide
chloro
儲存溫度
2-8°C
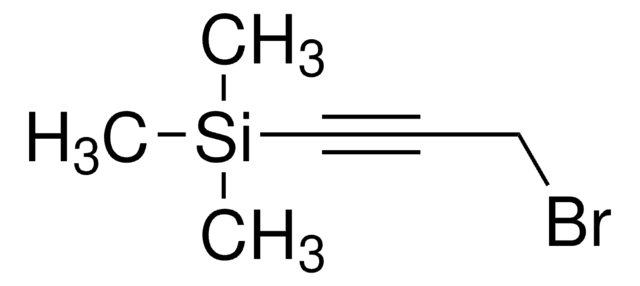
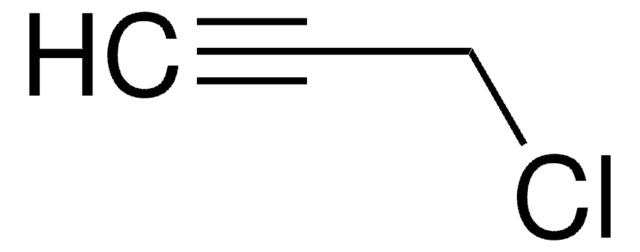
SMILES 字串
CC(C)(Cl)C#C
InChI
1S/C5H7Cl/c1-4-5(2,3)6/h1H,2-3H3
InChI 密鑰
QSILYWCNPOLKPN-UHFFFAOYSA-N
應用
3-氯-3-甲基-1-丁炔被用于制备:
- 苯并[b]吡喃并[2,3-i]呫吨-6-酮和苯并[b]吡喃并[3,2-h]呫吨-7-酮系列
- 6-羟基-3,3-二甲基-3H,7H-苯并[a]吡喃并[3,2-h]呫吨-7-酮
- 5-羟基-2,2-二甲基-2H,6H-苯并[a]吡喃并[2,3-i]呫吨-6-酮
- 3,3-二甲基-3H苯并呋喃[3,2-f][1]-苯并吡喃
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
39.2 °F - closed cup
閃點(°C)
4 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Chavalit Sittisombut et al.
Chemical & pharmaceutical bulletin, 54(8), 1113-1118 (2006-08-02)
Condensation of 2-hydroxy-1-naphthalenecarboxylic acid with phloroglucinol afforded 9,11-dihydroxy-12H-benzo[a]xanthen-12-one (6). Construction of an additional dimethylpyran ring onto this skeleton, by alkylation with 3-chloro-3-methyl-1-butyne followed by Claisen rearrangement, gave access to 6-hydroxy-3,3-dimethyl-3H,7H-benzo[a]pyrano[3,2-h]xanthen-7-one (12) and 5-hydroxy-2,2-dimethyl-2H,6H-benzo[a]pyrano[2,3-i]xanthen-6-one (13), which were methylated into 6-methoxy-3,3-dimethyl-3H,7H-benzo[a]pyrano[3,2-h]xanthen-7-one (14)
Soizic Prado et al.
Bioorganic & medicinal chemistry, 14(15), 5423-5428 (2006-04-18)
Alkylation of 2-hydroxydibenzofuran with 3-chloro-3-methyl-1-butyne, followed by Claisen rearrangement, gave access to 3,3-dimethyl-3Hbenzofuro[3,2-f][1]-benzopyran. Several derivatives modified at the pyran 1,2-double bond were prepared, including the corresponding dihydro compound and (+/-)-cis-diol, which was converted into diacetate and cyclic carbonate upon acylation.
C Sittisombut et al.
Chemical & pharmaceutical bulletin, 49(6), 675-679 (2001-06-20)
Condensation of 3-hydroxy-2-naphthalenecarboxylic acid with phloroglucinol afforded 1,3-dihydroxy-12H-benzo[b]xanthen-12-one. Construction of an additional dimethylpyran ring onto this skeleton, by alkylation with 3-chloro-3-methyl-1-butyne followed by Claisen rearrangement, gave access to a series of benzo[b]pyrano[2,3-i]xanthen-6-ones and benzo[b]pyrano[3,2-h]xanthen-7-ones related to psorospermine and benzo[b]acronycine. In
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務