推薦產品
品質等級
化驗
98%
bp
275-276 °C (lit.)
mp
59-61 °C (lit.)
SMILES 字串
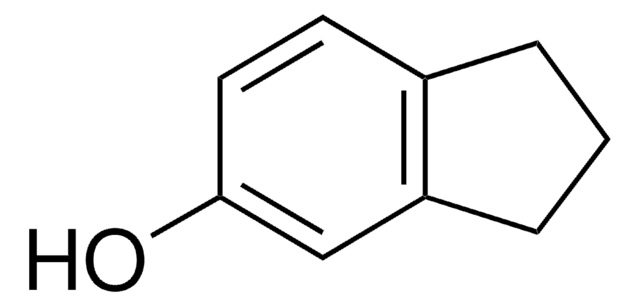
Oc1ccc2CCCCc2c1
InChI
1S/C10H12O/c11-10-6-5-8-3-1-2-4-9(8)7-10/h5-7,11H,1-4H2
InChI 密鑰
UMKXSOXZAXIOPJ-UHFFFAOYSA-N
應用
5,6,7,8-Tetrahydro-2-naphthol was used as a model compound in the study of photochemical transformation of 17β-estradiol (natural estrogenic steroid) and 17α-ethinylestradiol (synthetic oral contraceptive).
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
T Bhattacharya et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 59(3), 525-535 (2003-01-14)
Both steady state and time resolved spectroscopic measurements reveal that the prime process involved in quenching mechanism of the lowest excited singlet (S1) and triplet (T1) states of the well known electron acceptor 9-Cyanoanthracene (9CNA) in presence of 5,6,7,8-tetrahydro-1-naphthol (TH1N)
B Kalyanaraman et al.
The Journal of biological chemistry, 259(22), 14018-14022 (1984-11-25)
Electron spin resonance spectroscopy has been used to demonstrate production of semiquinone-free radicals from the oxidation of the catechol estrogens 2- and 4-hydroxyestradiol and 2,6- and 4,6-dihydroxyestradiol. Radicals were generated either enzymatically (using horseradish peroxidase-H2O2 or tyrosinase-O2) or by autoxidation
Patrick Mazellier et al.
Chemosphere, 73(8), 1216-1223 (2008-09-03)
The photochemical transformation of natural estrogenic steroid 17beta-estradiol (E2) and the synthetic oral contraceptive 17alpha-ethinylestradiol (EE2) has been studied in dilute non buffered aqueous solution (pH 5.5-6.0) upon monochromatic (254 nm) and polychromatic (lambda>290 nm) irradiation. Upon irradiation at 254
N M Howarth et al.
Steroids, 62(4), 346-350 (1997-04-01)
In our continuing quest to design efficient inhibitors of estrone sulfatase activity and to assess the recognition of estrone sulfate surrogates by estrone sulfatase, we synthesized and evaluated several sulfonate derivatives of 5,6,7,8-tetrahydronaphth-2-ol and estrone. 5,6,7,8-Tetrahydronaphth-2-methanesulfonate (11), and 5,6,7,8-tetrahydronaphth-2-(p-toluene)sulfonate (12)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務