全部照片(1)
About This Item
經驗公式(希爾表示法):
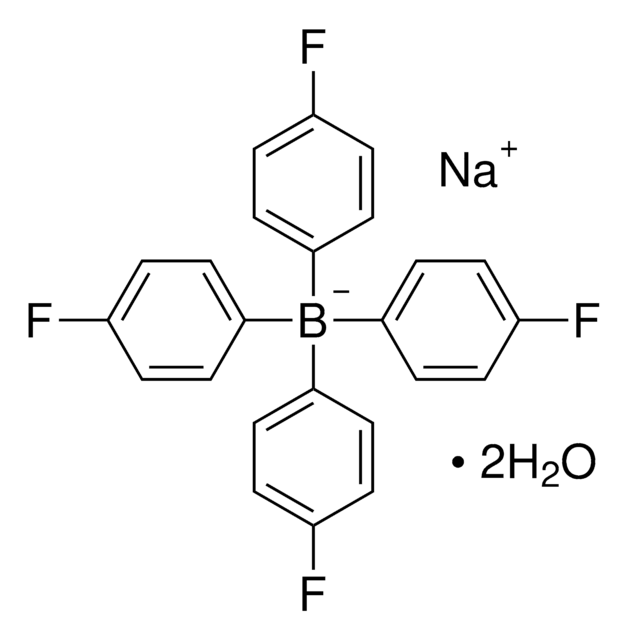
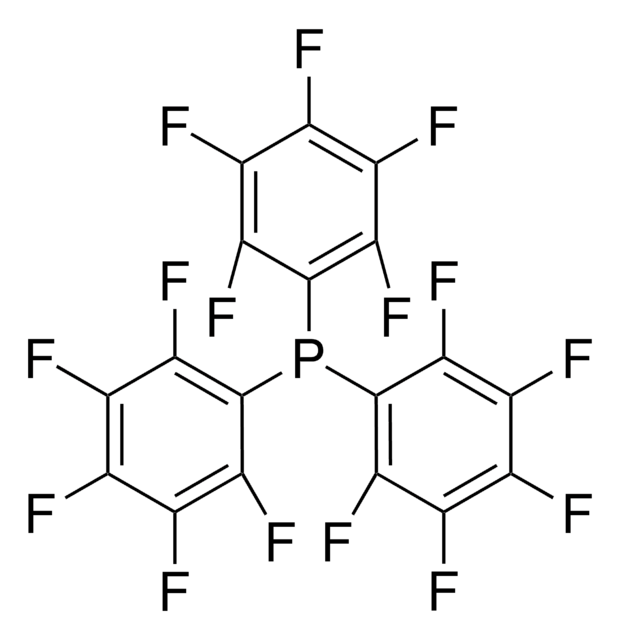
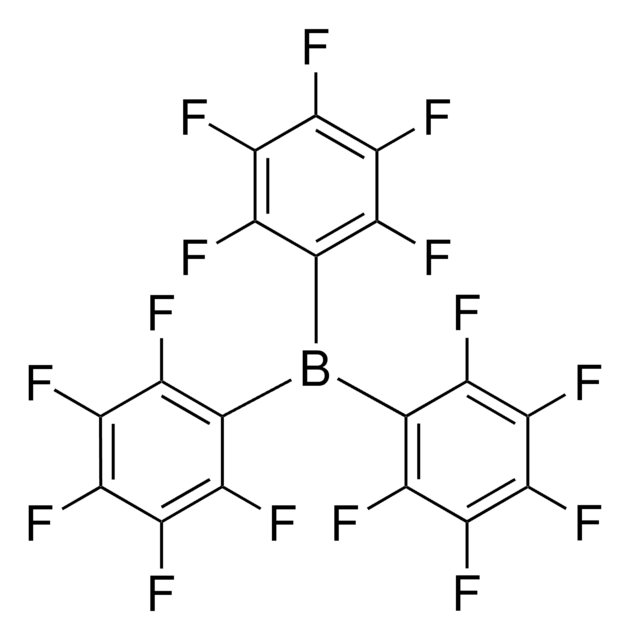
C24BF20Li · 2.5C4H10O
CAS號碼:
分子量::
871.28
MDL號碼:
分類程式碼代碼:
12161600
PubChem物質ID:
NACRES:
NA.22
推薦產品
反應適用性
core: boron
reagent type: catalyst
品質等級
mp
117-122 °C
SMILES 字串
[Li+].CCOCC.Fc1c(F)c(F)c(c(F)c1F)[B-](c2c(F)c(F)c(F)c(F)c2F)(c3c(F)c(F)c(F)c(F)c3F)c4c(F)c(F)c(F)c(F)c4F
InChI
1S/C24BF20.C4H10O.Li/c26-5-1(6(27)14(35)21(42)13(5)34)25(2-7(28)15(36)22(43)16(37)8(2)29,3-9(30)17(38)23(44)18(39)10(3)31)4-11(32)19(40)24(45)20(41)12(4)33;1-3-5-4-2;/h;3-4H2,1-2H3;/q-1;;+1
InChI 密鑰
KPLZKJQZPFREPG-UHFFFAOYSA-N
應用
四(五氟苯基)硼酸锂乙醚可用作:
- 电化学反应中与过渡金属催化剂配位的反离子,以增强其酸度或溶解度。
- 拜耳-维利格环烷酮氧化反应中的催化剂,以在双氧水和草酸存在下获得内酯。
- 合成聚(降冰片烯酯)的活化剂。
包裝
无底玻璃瓶。内含物装在插入式熔锥内。
其他說明
用于生成阳离子过渡金属络合物的盐
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
H. Shen, R.F. Jordan
Organometallics, 22, 1878-1878 (2003)
Kateryna Trofymchuk et al.
Nature photonics, 11(10), 657-663 (2017-10-07)
Here, we explore the enhancement of single molecule emission by polymeric nano-antenna that can harvest energy from thousands of donor dyes to a single acceptor. In this nano-antenna, the cationic dyes are brought together in very close proximity using bulky
High oxidation-state (formally d(0)) tungsten silylene complexes via double Si-H bond activation.
B V Mork et al.
Journal of the American Chemical Society, 123(39), 9702-9703 (2001-09-27)
Lucie Rivier et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(55), 12769-12779 (2019-07-10)
Detailed studies on hydrogen evolution by decamethylruthenocene ([Cp*2 RuII ]) highlighted that metallocenes are capable of photoreducing hydrogen without the need for an additional sensitizer. Electrochemical, gas chromatographic, and spectroscopic (UV/Vis, 1 H and 13 C NMR) measurements corroborated by DFT
Shu-Juan Liu et al.
Scientific reports, 7, 46669-46669 (2017-04-25)
Electrochemistry methods have been widely employed in the development of renewable energy, and involved in various processes, e.g. water splitting and oxygen reduction. Remarkable progress notwithstanding, there are still many challenges in further optimization of catalysts to achieve high performance.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![四 [3,5-双(三氟甲基)苯基] 硼酸钠](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)
![四[3,5-二(三氟甲基)苯基]硼酸钾 Selectophore™](/deepweb/assets/sigmaaldrich/product/structures/631/130/b5486f44-2e69-40d0-902f-dd71894a6add/640/b5486f44-2e69-40d0-902f-dd71894a6add.png)