推荐产品
等級
pharmaceutical primary standard
API 家族
gemcitabine
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
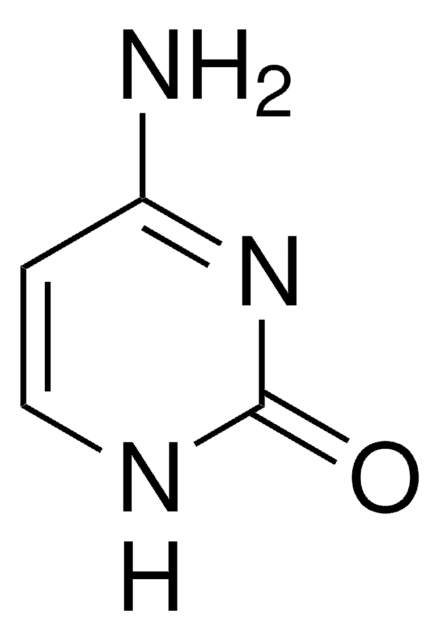
Cl.NC1=NC(=O)N(C=C1)[C@@H]2O[C@H](CO)[C@@H](O)C2(F)F
InChI
1S/C9H11F2N3O4.ClH/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17;/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17);1H/t4-,6-,7-;/m1./s1
InChI 密鑰
OKKDEIYWILRZIA-OSZBKLCCSA-N
基因資訊
human ... POLA1(5422) , POLA2(23649) , POLD1(5424) , POLD2(5425) , POLD3(10714) , POLD4(57804) , POLE(5426) , POLE2(5427) , POLE3(54107) , PRIM1(5557) , PRIM2(5558) , RRM1(6240) , RRM2(6241) , RRM2B(50484)
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
也用于制备LC-UV法检测和杂质分析用标准溶液,用于下列美国药典(USP)专论中的药物分析:
- 盐酸吉西他滨
- 注射用吉西他滨
生化/生理作用
分析報告
其他說明
相關產品
訊號詞
Danger
危險聲明
危險分類
Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门