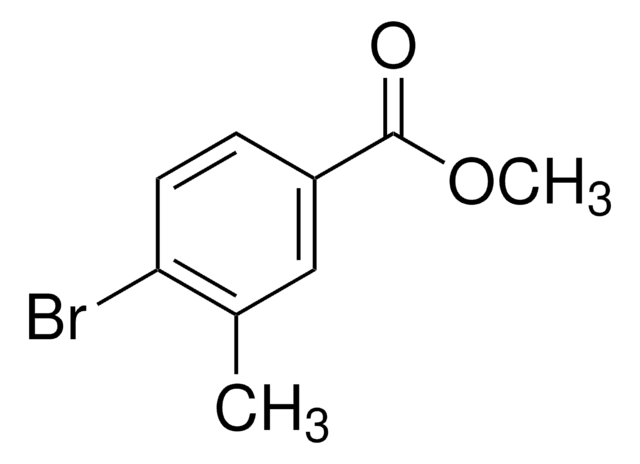
L511188
(S)-2-(2,3-Bis(dicyclohexylamino)cyclopropenimine)-3-phenylpropan-1-ol hydrochloride
AldrichCPR
别名:
(βS)-β-[[2,3-bis(dicyclohexylamino)-2-cyclopropen-1-ylidene]amino]-benzenepropanol hydrochloride (1:1), Dicyclohexyl cyclopropenimine, Lambert Cyclopropenimine Catalyst
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C36H56ClN3O
CAS号:
分子量:
582.30
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
推荐产品
SMILES 字串
Cl.OC[C@H](Cc1ccccc1)\N=C2\C(N(C3CCCCC3)C4CCCCC4)=C2N(C5CCCCC5)C6CCCCC6
InChI
1S/C36H55N3O.ClH/c40-27-29(26-28-16-6-1-7-17-28)37-34-35(38(30-18-8-2-9-19-30)31-20-10-3-11-21-31)36(34)39(32-22-12-4-13-23-32)33-24-14-5-15-25-33;/h1,6-7,16-17,29-33,40H,2-5,8-15,18-27H2;1H/t29-;/m0./s1
InChI 密鑰
RTCSAEOYHXMTHG-JMAPEOGHSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Learn More at the Professor and Product Portal of Professor Tristan Lambert.
應用
Chiral cyclopropenimines are highly effective new class of enantioselective Brønsted base catalysts, the so-called “superbases” for enantioselective organocatalysis.
Due to the prevalence of chemical reactions involving proton transfer as a key mechanistic event, Brønsted bases have become indispensable tools for the practice of organic synthetic chemistry, capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products.
Novel Brønsted bases provide potent yet tunable basicity to the acidity of a given substrate, are trivial to prepare, and offer unique opportunities for asymmetric transition state organization. The high basicity should allow them to catalyze a wide range of reactions (i.e. chiral pharmaceutical ingredients) and could lead to easier and faster syntheses of novel chiral compounds for drug discovery and other applications.
2,3-bis(dialkylamino)cyclopropenimines serve as a highly effective platform for chiral Brønsted base catalysis. Chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. Catalysis with chiral cyclopropenimines should be amenable to relatively large-scale applications under mild reaction conditions.

Due to the prevalence of chemical reactions involving proton transfer as a key mechanistic event, Brønsted bases have become indispensable tools for the practice of organic synthetic chemistry, capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products.
Novel Brønsted bases provide potent yet tunable basicity to the acidity of a given substrate, are trivial to prepare, and offer unique opportunities for asymmetric transition state organization. The high basicity should allow them to catalyze a wide range of reactions (i.e. chiral pharmaceutical ingredients) and could lead to easier and faster syntheses of novel chiral compounds for drug discovery and other applications.
2,3-bis(dialkylamino)cyclopropenimines serve as a highly effective platform for chiral Brønsted base catalysis. Chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. Catalysis with chiral cyclopropenimines should be amenable to relatively large-scale applications under mild reaction conditions.

其他說明
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jeffrey S Bandar et al.
Journal of the American Chemical Society, 134(12), 5552-5555 (2012-03-16)
Cyclopropenimines are shown to be a highly effective new class of enantioselective Brønsted base catalysts. A chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. A preparative scale reaction to deliver 25
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门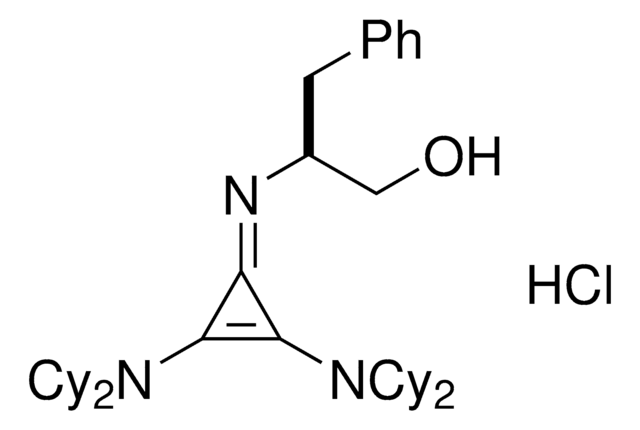

![N-[(1R,2R)-2-(1-哌啶基)环己基]-N′-[4-(三氟甲基)苯基]四酰胺 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)