所有图片(1)
About This Item
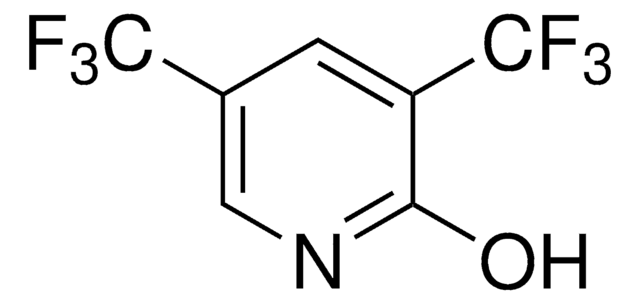
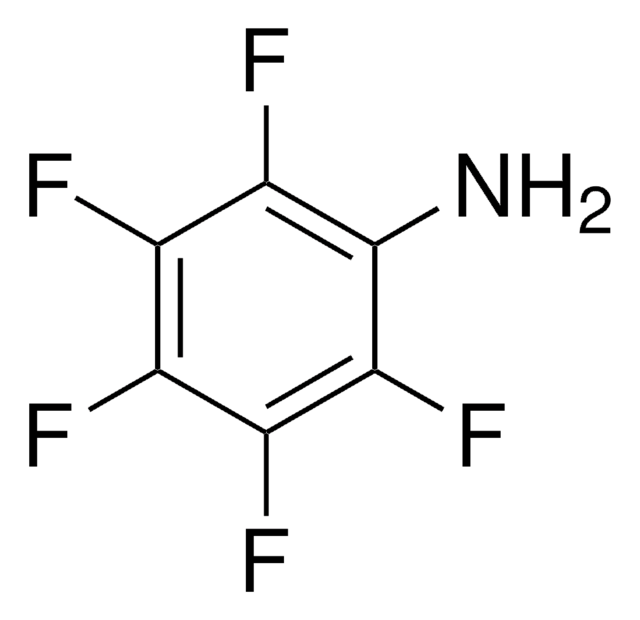
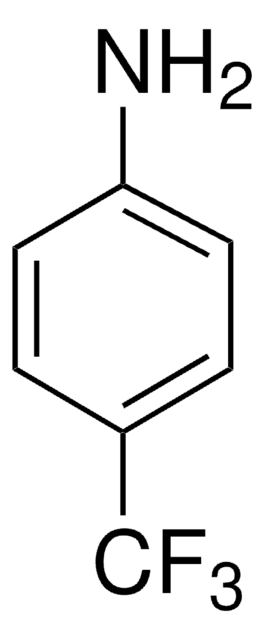
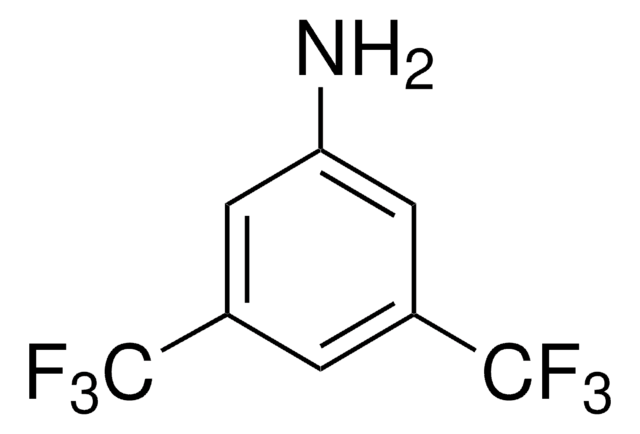
线性分子式:
CF3C6F4NH2
CAS号:
分子量:
233.09
Beilstein:
2657893
MDL號碼:
分類程式碼代碼:
12352101
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
形狀
liquid
反應適用性
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
折射率
n20/D 1.431 (lit.)
n20/D 1.432
bp
186 °C (lit.)
密度
1.662 g/mL at 25 °C
1.687 g/mL at 25 °C (lit.)
官能基
fluoro
SMILES 字串
Nc1c(F)c(F)c(c(F)c1F)C(F)(F)F
InChI
1S/C7H2F7N/c8-2-1(7(12,13)14)3(9)5(11)6(15)4(2)10/h15H2
InChI 密鑰
FJOACTZFMHZHSC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
其他說明
Acidic amides are superior directing groups for promoting C-H activation reactions with both Pd(0)/PR3 and Pd(II) catalysts.
Used in the Preparation of
Used in the Preparation of
- Lactams via palladium-catalyzed olefination of arylamides with benzylacrylate, followed by 1,4-conjugate addition
- N-(fluorinated aryl)benzamides as substrates for regioselective C-H amination reactions with O-benzoylhydroxylamines
- Substituted succinimides via palladium-catalyzed carbonylation of N-aryl amides
- N-aryl cyclopropanecarboxamide substrates and various amino acid ligands for palladium-catalyzed C-H activation of cyclopropanes
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Wasa, M;
Journal of the American Chemical Society, 132(11), 3680-3681 null
Palladium(0)-Catalyzed Alkynylation of C(sp3)-H Bonds
He, J.:
Journal of the American Chemical Society null
Eun Jeong Yoo et al.
Journal of the American Chemical Society, 133(20), 7652-7655 (2011-04-28)
C-H amination of N-aryl benzamides with O-benzoyl hydroxylamines has been achieved with either Pd(II) or Pd(0) catalysts. Furthermore, we demonstrate that secondary amines can be directly used with benzoyl peroxide in a one-pot procedure that proceeds via the in situ
Eun Jeong Yoo et al.
Journal of the American Chemical Society, 132(49), 17378-17380 (2010-11-19)
Pd(II)-catalyzed β-C(sp(3))-H carbonylation of N-arylamides under CO (1 atm) has been achieved. Following amide-directed C(sp(3))-H cleavage and insertion of CO into the resulting [Pd(II)-C(sp(3))] bond, intramolecular C-N reductive elimination gave the corresponding succinimides, which could be readily converted to 1,4-dicarbonyl
Masayuki Wasa et al.
Journal of the American Chemical Society, 133(49), 19598-19601 (2011-11-09)
Systematic ligand development has led to the identification of novel mono-N-protected amino acid ligands for Pd(II)-catalyzed enantioselective C-H activation of cyclopropanes. A diverse range of organoboron reagents can be used as coupling partners, and the reaction proceeds under mild conditions.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门