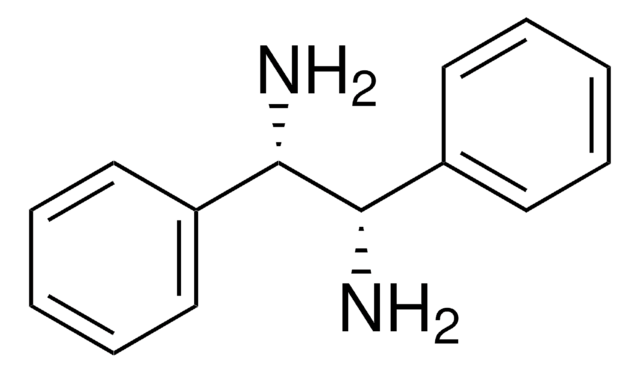
458511
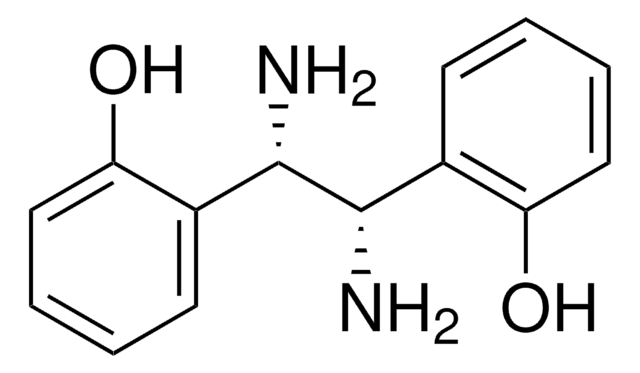
内消旋-1,2-二苯基乙二胺
98%
别名:
(1R,2S)-Diaminodiphenylethane, meso-1,2-Diamino-1,2-diphenylethane, meso-1,2-Diphenyl-1,2-ethanediamine, meso-1,2-Diphenyl-1,2-ethylenediamine, meso-1,2-Diphenyldiaminoethane, meso-1,2-Diphenylethanediamine, meso-Stilbenediamine, rel-(1R,2S)-1,2-Diphenyl-1,2-ethanediamine
登录查看公司和协议定价
所有图片(1)
About This Item
线性分子式:
[C6H5CH(NH2)-]2
CAS号:
分子量:
212.29
MDL號碼:
分類程式碼代碼:
12352100
eCl@ss:
39011513
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
98%
mp
118-122 °C (lit.)
SMILES 字串
N[C@H]([C@H](N)c1ccccc1)c2ccccc2
InChI
1S/C14H16N2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14H,15-16H2/t13-,14+
InChI 密鑰
PONXTPCRRASWKW-OKILXGFUSA-N
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Koichi Kodama et al.
Organic & biomolecular chemistry, 10(9), 1877-1882 (2012-01-26)
A supramolecular chiral host consisting of N-(2-naphthoyl)-L-aspartic acid (L-1) and meso-1,2-diphenylethylenediamine (2) is effective in enantioseparation of 1-arylethanols (up to 96% ee with 100% inclusion ratio). Here we report three different methods to prepare the inclusion crystals and discuss the
Nobuko Mibu et al.
Chemical & pharmaceutical bulletin, 56(7), 1052-1058 (2008-07-02)
N-carbamoyl and N-acyl diamine derivatives were synthesized from symmetrical diamines by their addition to iso(thio)cyanates, cleavage reaction of acid anhydride, or N-acylation by acyl chloride. (1R,2R)-1,2-Diaminocyclohexane [(1R,2R)-1], meso-1,2-diaminocyclohexane (meso-1), (1R,2R)-1,2-diphenylethylenediamine [(1R,2R)-3], or meso-1,2-diphenylethylenediamine (meso-3) were used as the starting symmetrical
Stereoselective Synthesis of (1R, 2S, 3R)-Camphordiamine.
Busacca CA, et al.
The Journal of Organic Chemistry, 65(15), 4753-4755 (2000)
Christian Rummey et al.
Bioorganic & medicinal chemistry letters, 16(5), 1405-1409 (2005-12-03)
Dipeptidyl peptidase IV is a clinically validated target for type-2 diabetes and belongs to a family of peptidases with a quite unique post-proline cleavage specificity. Known inhibitors contain a limited number of molecular anchors occupying the small prototypical S1 pocket.
Irina Veselova et al.
Talanta, 171, 108-114 (2017-05-30)
The paper presents a novel multi-purpose enzymatic system and procedures for fluorescent determination of several flavonoids in herbal pharmaceuticals and plant materials after their enzyme-catalyzed oxidation by hydrogen peroxide and further derivatization with meso-1,2-diphenylethylenediamine. This system may be used for
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门