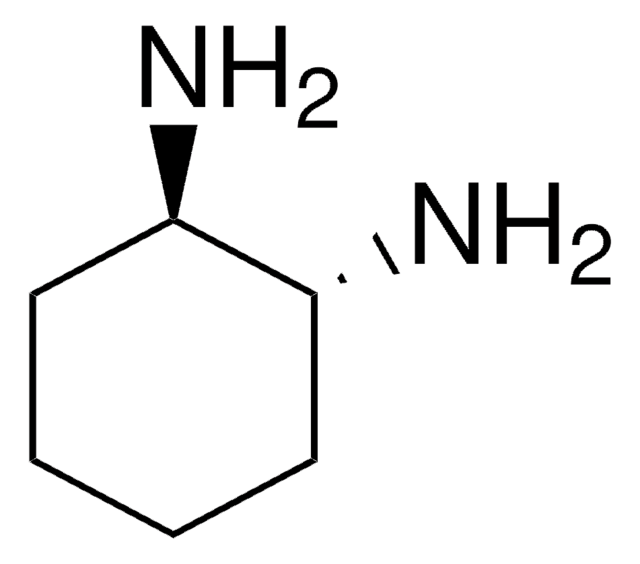
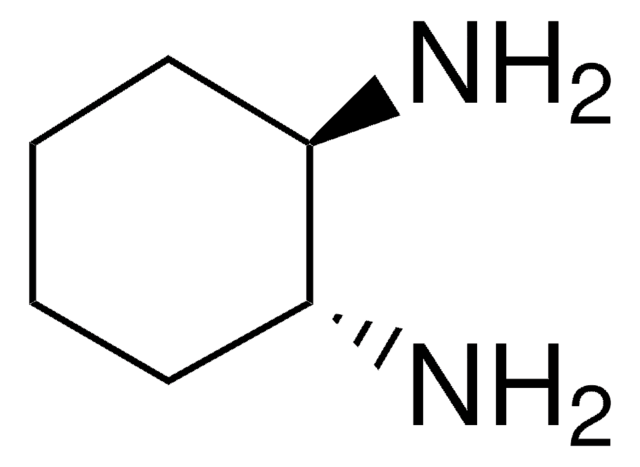
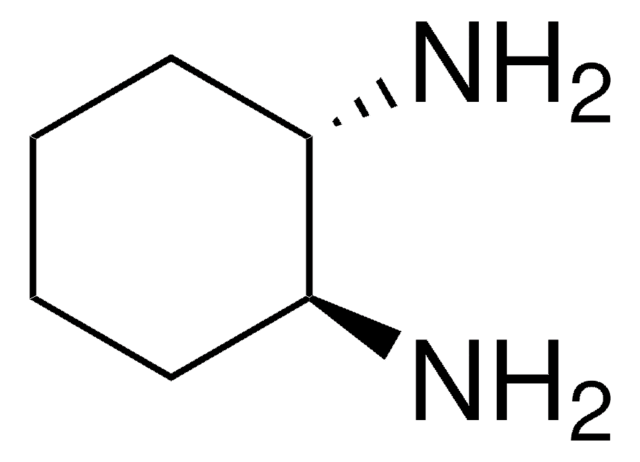
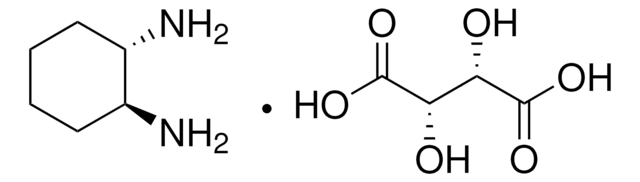
推荐产品
蒸汽壓力
0.4 mmHg ( 20 °C)
化驗
99%
形狀
liquid
折射率
n20/D 1.49 (lit.)
bp
92-93 °C/18 mmHg (lit.)
密度
0.931 g/mL at 25 °C (lit.)
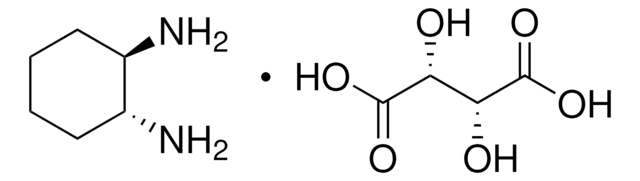
SMILES 字串
NC1CCCCC1N
InChI
1S/C6H14N2/c7-5-3-1-2-4-6(5)8/h5-6H,1-4,7-8H2
InChI 密鑰
SSJXIUAHEKJCMH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
1,2-二氨基环己烷与同手性化合物以及外消旋形式的 反式 -1,2-二氨基环己烷与对苯二甲醛发生非模板反应,生成 (3 + 3)-环缩合分子三角形 。它作为配体与有机锡形成络合物,作为金属类抗肿瘤药物具有潜在的应用价值 。
應用
将 1,2-二氨基环己烷用于手性钌 (Ⅳ) 氧配合物的合成 。
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
158.0 °F - closed cup
閃點(°C)
70 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
(3+ 3)-Cyclocondensation of the enantiopure and racemic forms of trans-1, 2-diaminocyclohexane with terephthaldehyde. Formation of diastereomeric molecular triangles and their stereoselective solid-state stacking into microporous chiral columns.
Chadim M, et al.
Tetrahedron Asymmetry, 12(1), 127-133 (2001)
J J Bonire et al.
Journal of inorganic biochemistry, 83(2-3), 217-221 (2001-03-10)
Platinum compounds containing the ligand 1,2-diaminocyclohexane (DACH) such as tetraplatin [PtCl4(DACH)] have been found to be active in cisplatin-resistant tumour models. In an attempt to develop novel metal-based drugs with a different therapeutic profile to cisplatin, we have synthesised a
Chiral ruthenium (IV)-oxo complexes. Structure, reactivities of [Ru (terpy)(Nn N) O]2+Nn N= N, N, N', N'-tetramethyl-1, 2-diaminocyclohexane) and [Ru (Me3 tacn)(cbpy) O]2+ (cbpy=(-)-3, 3'-[(4 S-trans)-1, 3-dioxolane-4, 5-dimethyl]-2, 2'-bipyridine).
Cheng WC, et al.
Inorgorganica Chimica Acta, 242(1), 105-113 (1996)
Takuya Kurahashi et al.
Journal of the American Chemical Society, 131(34), 12394-12405 (2009-08-27)
A series of Mn(IV)(salen)(L)(2) complexes bearing different external axial ligands (L = Cl, NO(3), N(3), and OCH(2)CF(3)) from chiral salen ligands with trans-cyclohexane-1,2-diamine as a chiral scaffold are synthesized, to gain insight into conformational properties of metal salen complexes. X-ray
Kui Mei et al.
Organic letters, 11(13), 2864-2867 (2009-06-03)
A highly enantioselective conjugate addition of nitroalkanes to enones has been developed. The process is efficiently catalyzed by a simple chiral cyclohexanediamine-derived primary amine thiourea with a broad substrate scope.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门