所有图片(1)
About This Item
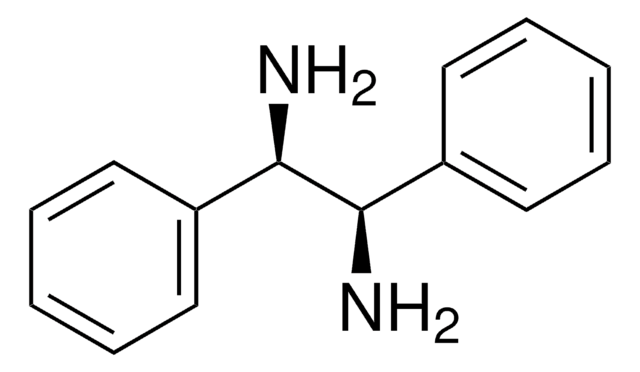
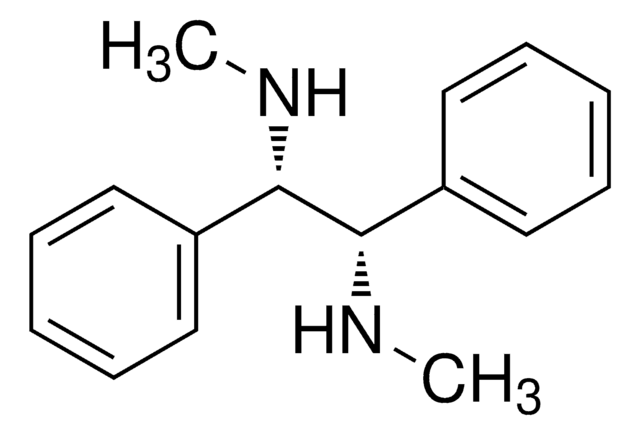
线性分子式:
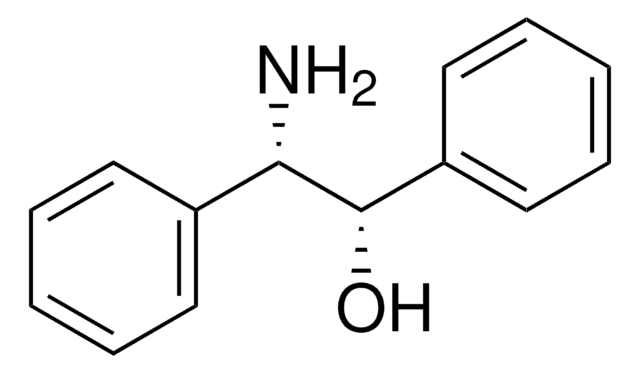
[C6H5CH(NH2)-]2
CAS号:
分子量:
212.29
Beilstein:
3201645
MDL號碼:
分類程式碼代碼:
12352116
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
光學活性
[α]20/D −102°, c = 1 in ethanol
光學純度
ee: 98% (GLC)
mp
83-85 °C (lit.)
官能基
amine
phenyl
SMILES 字串
N[C@H]([C@@H](N)c1ccccc1)c2ccccc2
InChI
1S/C14H16N2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14H,15-16H2/t13-,14-/m0/s1
InChI 密鑰
PONXTPCRRASWKW-KBPBESRZSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
1,2-二苯基乙二胺是一种手性分子,通常用作手性拆分剂和手性助剂前体。它还可用作NMR研究的手性溶剂化试剂。
應用
形成金属络合物的多功能配体。用于合成在不对称催化中具有应用潜力的 tropocoronand。
包裝
无底玻璃瓶。内含物在插入的融合锥体内。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
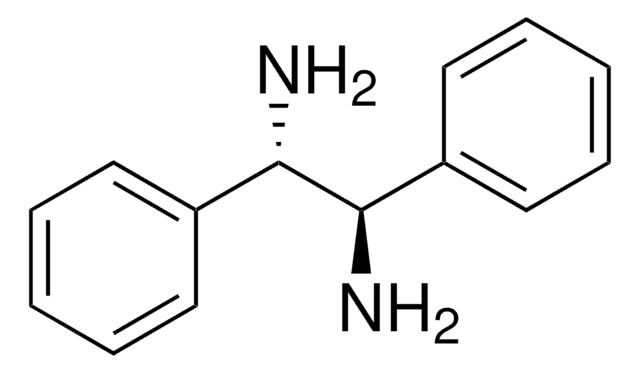
(S,S)-1,2-Diphenylethylenediamine
Chiral Reagents for Asymmetric Synthesis (2003)
Jérôme Long et al.
Science (New York, N.Y.), 367(6478), 671-676 (2020-02-08)
Magnetoelectric (ME) materials combine magnetic and electric polarizabilities in the same phase, offering a basis for developing high-density data storage and spintronic or low-consumption devices owing to the possibility of triggering one property with the other. Such applications require strong
Chenier, P.J. et al.
Tetrahedron Letters, 38, 7341-7341 (1997)
Mukaiyama, T.
Aldrichimica Acta, 29, 59-59 (1996)
N M Maier et al.
Chirality, 8(7), 490-493 (1996-01-01)
Fast and efficient baseline separation of asymmetrically substituted diarylmethanols and 1,1-diarylethanols was achieved on an endcapped, amide-linked N-3,5-dinitrobenzoylated, (R,R)-1,2-diphenyl-1,2-ethanediamine-derived chiral stationary phase (CSP). Optimal enantioselectivities on this CSP were obtained using 1% 2-propanol in n-heptane as the mobile phase. Enantiorecognition
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持