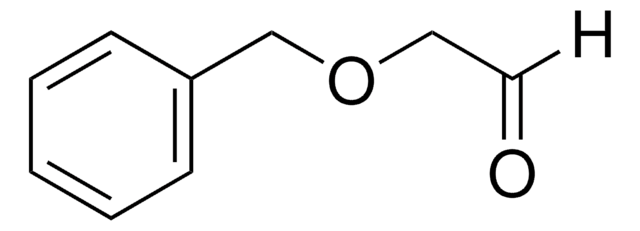
449458
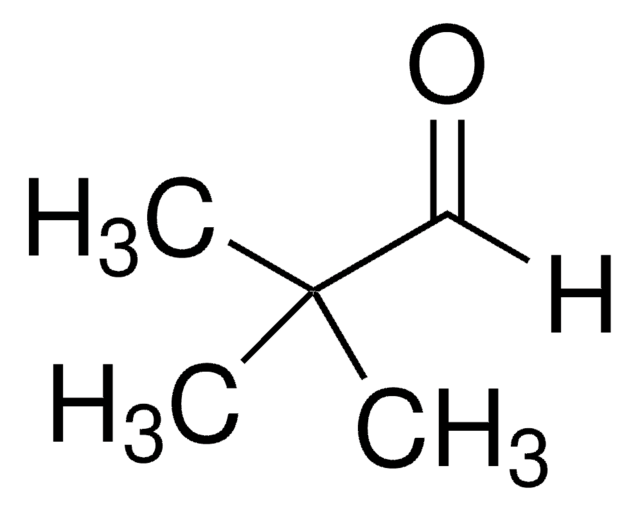
(叔丁基二甲基硅氧基)乙醛
90%
别名:
(tert-Butyldimethylsiloxy)acetaldehyde, 2-(tert-Butyldimethylsilyloxy)acetaldehyde, 2-[(tert-Butyl)dimethylsiloxy]acetaldehyde, 2-[(tert-Butyldimethylsilanyl)oxy]acetaldehyde, 2-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]acetaldehyde, Dimethyl-tert-butylsilyloxyacetaldehyde
登录查看公司和协议定价
所有图片(3)
About This Item
线性分子式:
(CH3)3CSi(CH3)2OCH2CHO
CAS号:
分子量:
174.31
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
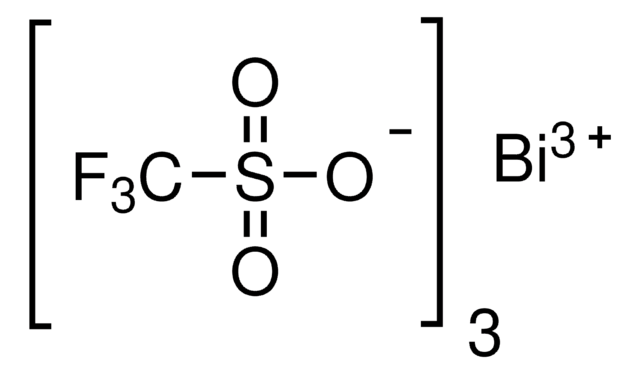
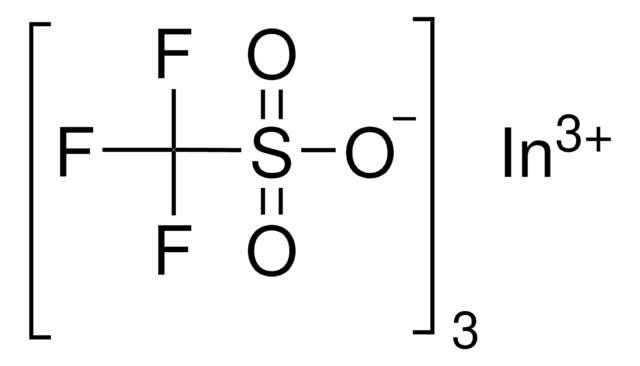
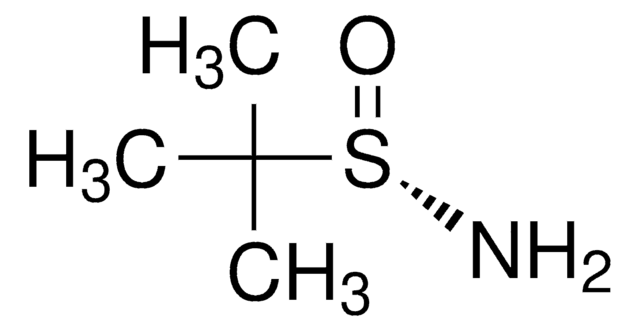
推荐产品
化驗
90%
折射率
n20/D 1.432 (lit.)
bp
165-167 °C (lit.)
密度
0.915 g/mL at 25 °C (lit.)
官能基
aldehyde
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)[Si](C)(C)OCC=O
InChI
1S/C8H18O2Si/c1-8(2,3)11(4,5)10-7-6-9/h6H,7H2,1-5H3
InChI 密鑰
MEBFFOKESLAUSJ-UHFFFAOYSA-N
相关类别
應用
(tert-Butyldimethylsilyloxy)acetaldehyde is a versatile reagent commonly used in synthetic glycobiology. It can act both as an aldol donor and an aldol acceptor in the stereocontrolled production of erythrose. It is used as an important reagent in the total synthesis of (+)-ambruticin, (−)-laulimalide, (−)-salinosporamide A, and (+)-leucascandrolide A.
Employed in the construction of the key tetrahydropyran subunit in a recent synthesis of the marine natural product (–)-dactylodide.
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
140.0 °F - closed cup
閃點(°C)
60 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Colobert, Francoise; et al.
European Journal of Organic Chemistry, 6, 1455-1467 (2006)
Total synthesis of the microtubule-stabilizing agent (−)-Laulimalide
Paterson I, et al.
Organic Letters, 3(20), 3149-3152 (2001)
Stereocontrolled total synthesis of (+)-leucascandrolide A.
Paterson I and Tudge M
Angewandte Chemie (International Edition in English), 115(3), 357-361 (2003)
Entry to Heterocycles Based on Indium-Catalyzed Conia-Ene Reactions: Asymmetric Synthesis of (−)-Salinosporamide A.
Takahashi K, et al.
Angewandte Chemie (International Edition in English), 47(33), 6244-6246 (2008)
Total synthesis of (+)-ambruticin.
Liu P and Jacobsen EN
Journal of the American Chemical Society, 123(43), 10772-10773 (2001)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门