所有图片(2)
About This Item
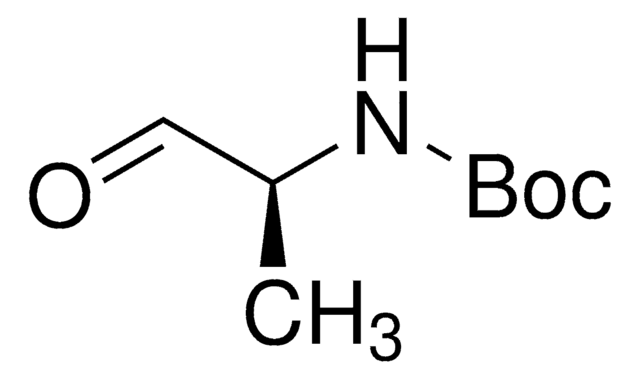
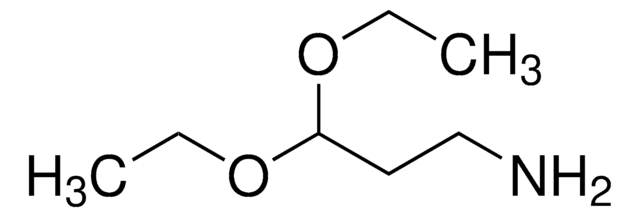
线性分子式:
HCOCH2NHCO2C(CH3)3
CAS号:
分子量:
159.18
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
95%
折射率
n20/D 1.455 (lit.)
儲存溫度
−20°C
SMILES 字串
CC(C)(C)OC(=O)NCC=O
InChI
1S/C7H13NO3/c1-7(2,3)11-6(10)8-4-5-9/h5H,4H2,1-3H3,(H,8,10)
InChI 密鑰
ACNRTYKOPZDRCO-UHFFFAOYSA-N
基因資訊
human ... CTSK(1513)
相关类别
一般說明
N -Boc-2-氨基乙醛是一种有机构件。与 Horner-Wadsworth-Emmons (HWE) 试剂反应,得到 γ-氨基丁酸 (GABA) 衍生的 α-酮酰胺/酯单元。
應用
N -Boc-2-氨基乙醛可用于以下用途:
- 作为 (+)-负霉素全合成中的起始试剂。
- ( E )-4-(( 叔 -丁氧基羰基)氨基)-2-烯酸乙酯的合成。
- 2,2′ 的合成-联吡啶。
使用最近报道的涉及甲醛和吡咯烷丙酸或二肽 L-Pro-β-Ala 催化的方案,该氨基醛的 α-亚甲基化以快速有效的方式进行。
也用于含 1,3-偶极环加成的吡咯烷的三组分合成。
也用于含 1,3-偶极环加成的吡咯烷的三组分合成。
受保护的吡咯脯氨酸合成中的一个组成部分。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Anniina Erkkilä et al.
The Journal of organic chemistry, 71(6), 2538-2541 (2006-03-11)
A rapid and extremely convenient method for alpha-methylenation of aldehydes with aqueous formaldehyde is described. Two optimal catalytic systems are presented that allow short reaction times and afford the functionalized products in good to excellent yields (up to 99%) and
Anna Turetsky et al.
Scientific reports, 4, 4782-4782 (2014-04-25)
A number of Bruton's tyrosine kinase (BTK) inhibitors are currently in development, yet it has been difficult to visualize BTK expression and pharmacological inhibition in vivo in real time. We synthesized a fluorescent, irreversible BTK binder based on the drug
Diethyl [3-Cyano-2-Oxo-3-(Triphenylphosphoranylidene) propyl] phosphonate: A Useful Horner-Wadsworth-Emmons Reagent for alpha-Keto (Cyanomethylene)-triphenylphosphoranes from Carbonyl Compounds.
Lee K.
Bull. Korean Chem. Soc., 28(10), 1641-1641 (2007)
Claudia Karnthaler-Benbakka et al.
Angewandte Chemie (International ed. in English), 53(47), 12930-12935 (2014-08-01)
The development of receptor tyrosine-kinase inhibitors (TKIs) was a major step forward in cancer treatment. However, the therapy with TKIs is limited by strong side effects and drug resistance. The aim of this study was the design of novel epidermal
The Journal of Organic Chemistry, 70, 10869-10869 (2005)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门