所有图片(2)
About This Item
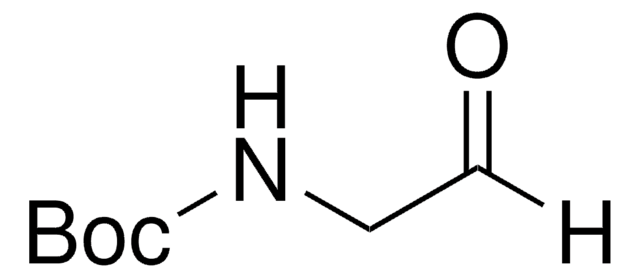
经验公式(希尔记法):
C8H15NO3
CAS号:
分子量:
173.21
MDL號碼:
分類程式碼代碼:
12352209
PubChem物質ID:
NACRES:
NA.26
推荐产品
product name
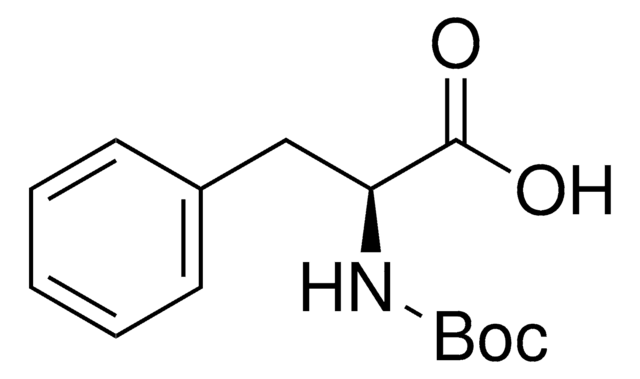
Boc-L-alaninal, ≥98%
品質等級
化驗
≥98%
形狀
powder
顏色
white
應用
peptide synthesis
儲存溫度
−20°C
SMILES 字串
C[C@H](NC(=O)OC(C)(C)C)C=O
InChI
1S/C8H15NO3/c1-6(5-10)9-7(11)12-8(2,3)4/h5-6H,1-4H3,(H,9,11)/t6-/m0/s1
InChI 密鑰
OEQRZPWMXXJEKU-LURJTMIESA-N
基因資訊
human ... CTSK(1513)
應用
Boc-L-alaninal is used as a reagent for organic synthesis of C(26)-C(32) Oxazole Fragment of Calyculin C and other molecules.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Petri M. Pihko et al.
The Journal of organic chemistry, 63(1), 92-98 (2001-10-25)
The synthesis of the C(26)-C(32) oxazole fragment 4 and its C(32) epimer 20 of serine/threonine protein phosphatase PP1 and PP2A inhibitor calyculin C is presented. The syn methyl arrangement in 4 was established through cyclic stereocontrol. Several methods for oxidizing
Johann Chan et al.
The Journal of organic chemistry, 76(6), 1767-1774 (2011-02-09)
Two new, reliable syntheses of a pyrido[2,3-d]-pyrimidine inhibitor of the CXCR3 receptor are described. A nine-step synthesis of the CXCR3 inhibitor (1) from 2-aminonicotinic acid was demonstrated on a multikilogram scale and incorporates a classic resolution to deliver the enantioenriched
James A Marshall et al.
Organic letters, 7(8), 1593-1596 (2005-04-09)
[reaction: see text] Additions of chiral allenylzinc and indium reagents to N-Boc alaninal were examined as a possible route to a C20-C26 segment of superstolide A. Allenylzinc reagents, prepared in situ by palladiozincation of (R)- and (S)-5-pivalyloxy-3-butyn-2-ol mesylate, showed excellent
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门