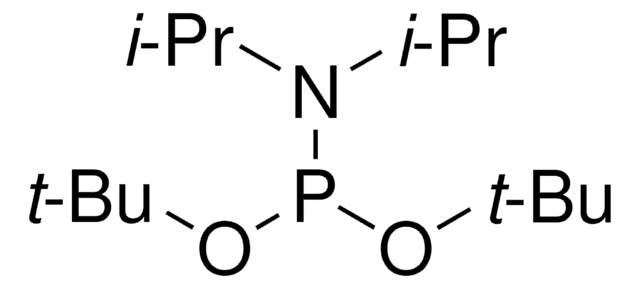
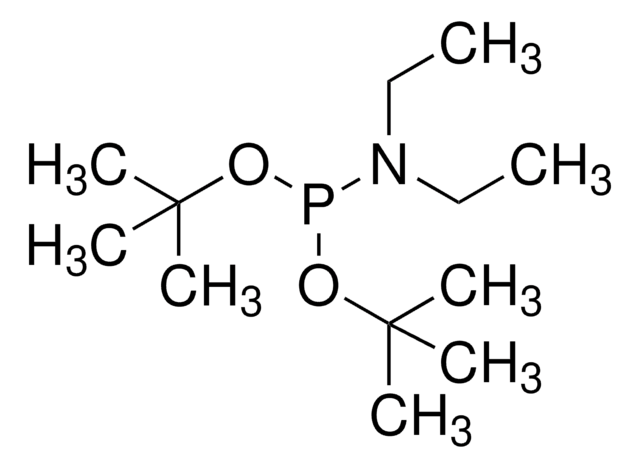
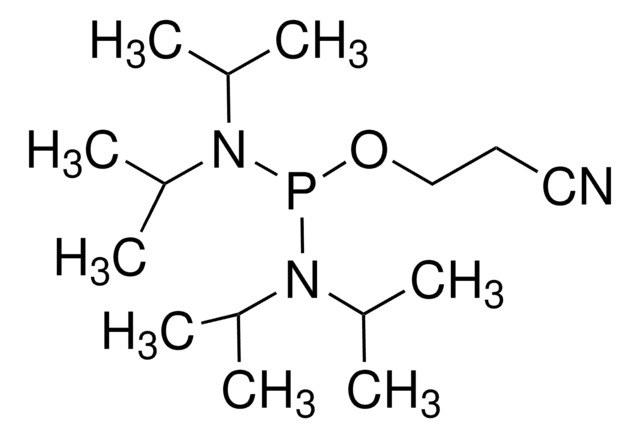
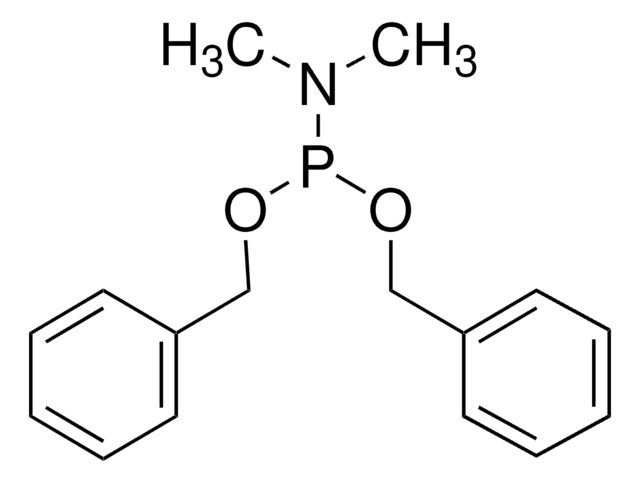
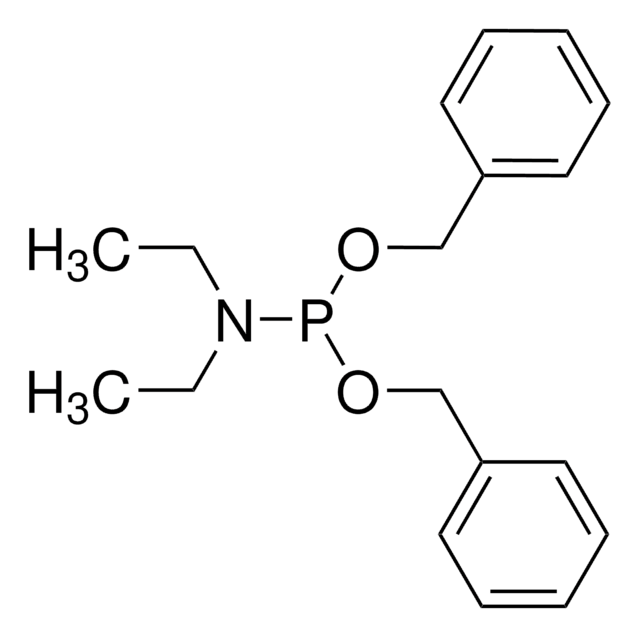
推荐产品
等級
technical grade
品質等級
化驗
90%
形狀
liquid
折射率
n20/D 1.535 (lit.)
bp
130 °C/0.55 mmHg (lit.)
溶解度
THF: soluble(lit.)
acetonitrile: soluble(lit.)
cold water: insoluble(lit.)
dichloromethane: soluble(lit.)
密度
1.028 g/mL at 25 °C (lit.)
官能基
amine
phenyl
儲存溫度
2-8°C
SMILES 字串
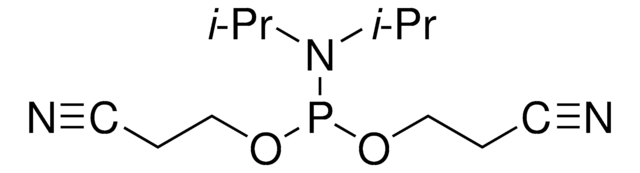
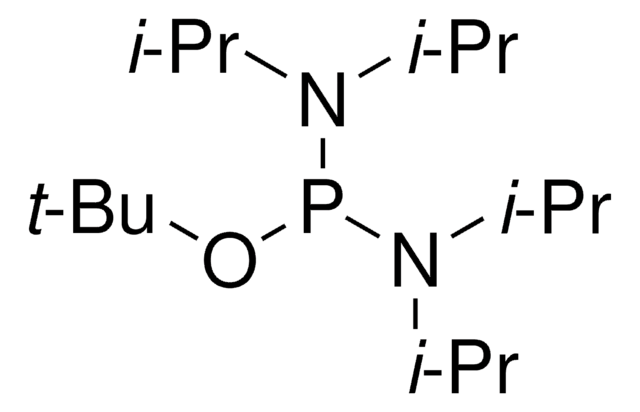
CC(C)N(C(C)C)P(OCc1ccccc1)OCc2ccccc2
InChI
1S/C20H28NO2P/c1-17(2)21(18(3)4)24(22-15-19-11-7-5-8-12-19)23-16-20-13-9-6-10-14-20/h5-14,17-18H,15-16H2,1-4H3
InChI 密鑰
ANPWLBTUUNFQIO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
N,N-二异丙基亚磷酰胺基二苄基酯可用于制备磷酸肽。它可用于合成GDP(鸟苷二磷酸)类似物SML-8-73-1。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
156.2 °F - closed cup
閃點(°C)
69 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
D M Andrews et al.
International journal of peptide and protein research, 38(5), 469-475 (1991-11-01)
A completely general method for the O-phosphorylation of peptides of any given composition using solid-phase methodology is described. Peptides were assembled using Fmoc amino acid active esters, with base used for Fmoc deprotection. Unprotected amino acid side chain hydroxyl groups
D L Clemm et al.
Molecular endocrinology (Baltimore, Md.), 14(1), 52-65 (2000-01-11)
Human progesterone receptor (PR) is phosphorylated on multiple serine residues (at least seven sites) in a manner that involves distinct groups of sites coordinately regulated by hormone and different kinases. Progress on defining a functional role for PR phosphorylation has
Synthesis, 9, 1481-1485 (2004)
Jie Xue et al.
Organic letters, 6(9), 1365-1368 (2004-04-23)
[reaction: see text] A new one-step reaction has been developed for converting 4-azido-4-deoxy-d-galactoside into 4-deoxy-d-erythro-hexos-3-ulose by phosphoramidites and tetrazole. It is proposed that the new reaction proceeds via an intramolecular Staudinger reaction of the phosphite intermediate and a tetrazole-catalyzed elimination
Sang Min Lim et al.
Angewandte Chemie (International ed. in English), 53(1), 199-204 (2013-11-22)
We report the synthesis of a GDP analogue, SML-8-73-1, and a prodrug derivative, SML-10-70-1, which are selective, direct-acting covalent inhibitors of the K-Ras G12C mutant relative to wild-type Ras. Biochemical and biophysical measurements suggest that modification of K-Ras with SML-8-73-1
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门