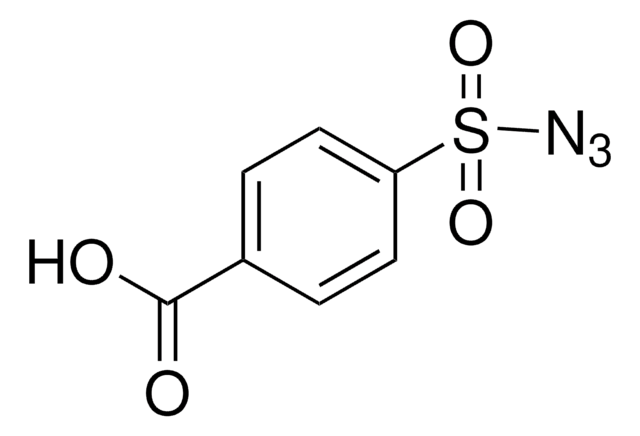
推荐产品
品質等級
化驗
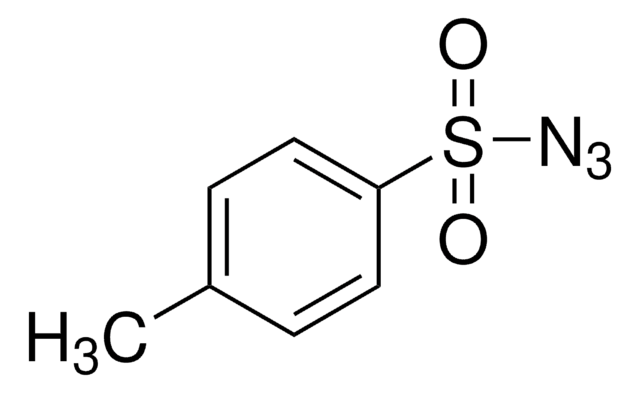
97%
形狀
solid
反應適用性
reaction type: click chemistry
mp
107-111 °C (lit.)
官能基
amide
azide
SMILES 字串
CC(=O)Nc1ccc(cc1)S(=O)(=O)N=[N+]=[N-]
InChI
1S/C8H8N4O3S/c1-6(13)10-7-2-4-8(5-3-7)16(14,15)12-11-9/h2-5H,1H3,(H,10,13)
InChI 密鑰
NTMHWRHEGDRTPD-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
单糖衍生醇
非肽类 NK3 受体拮抗剂
试剂:
后期分子间 C-H 烯化反应
抗肿瘤药物的分子内异甲基丙酮环加成反应
铑催化乙烯磺酸酯的卡宾环合环加成反应级联反应
Suzuki-Miyaura 交叉偶联反应
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
商品
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
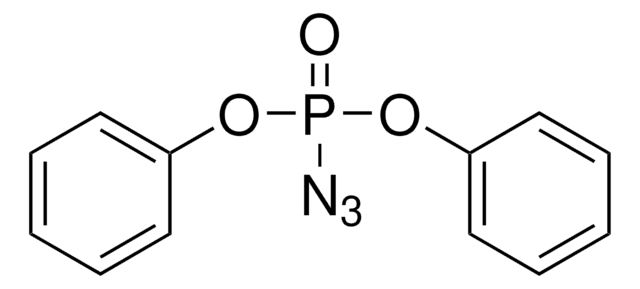

![1,4-二叠氮双环[2.2.2]辛烷 ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)