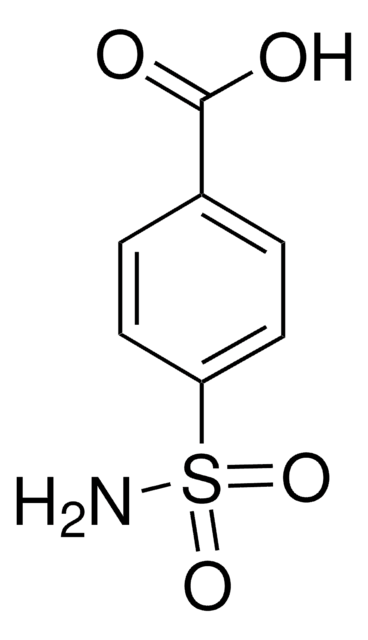
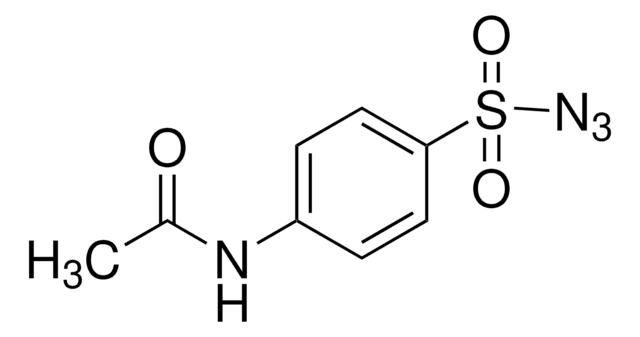
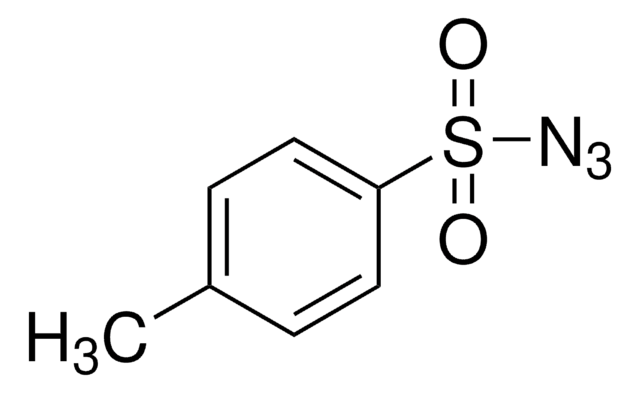
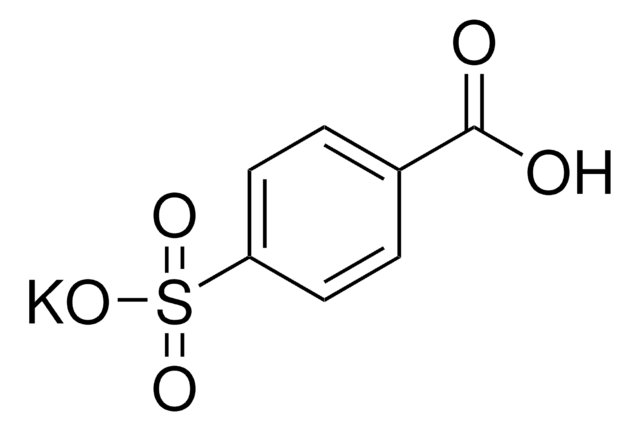
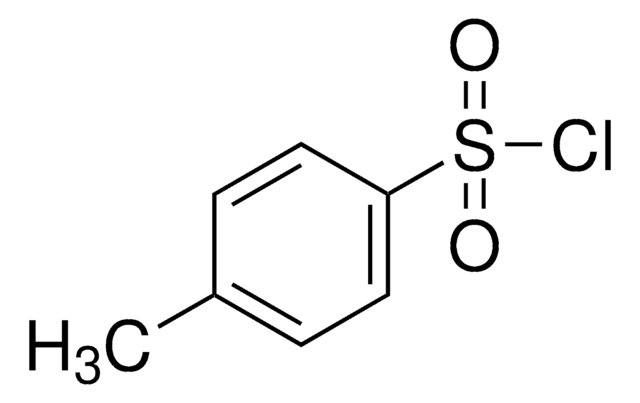
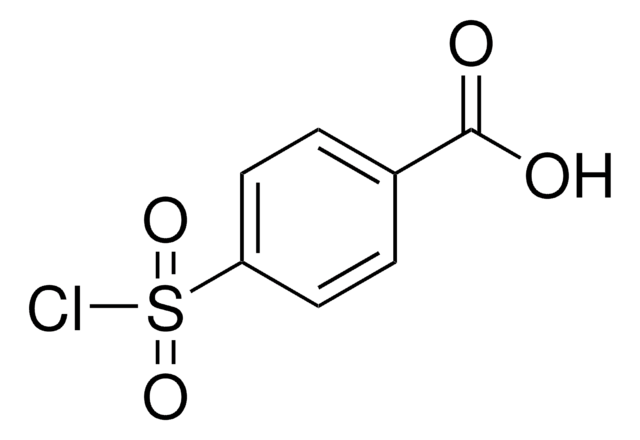
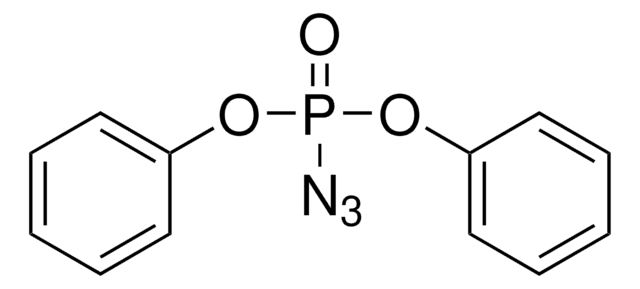
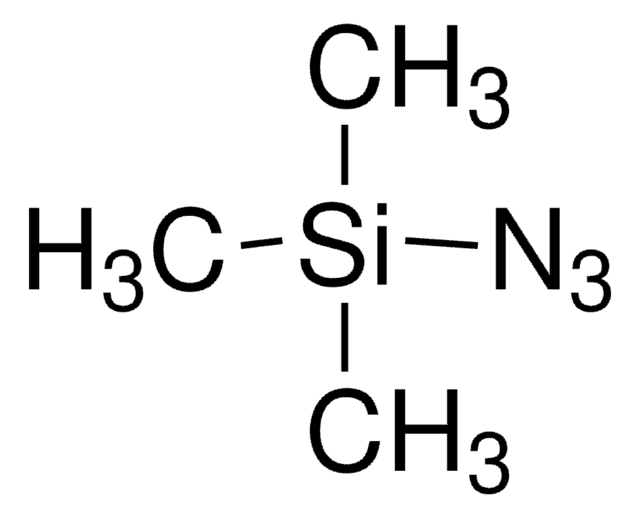About This Item
推荐产品
品質等級
化驗
97%
反應適用性
reaction type: click chemistry
mp
180 °C (dec.) (lit.)
儲存溫度
2-8°C
SMILES 字串
OC(=O)c1ccc(cc1)S(=O)(=O)N=[N+]=[N-]
InChI
1S/C7H5N3O4S/c8-9-10-15(13,14)6-3-1-5(2-4-6)7(11)12/h1-4H,(H,11,12)
InChI 密鑰
OWULJVXJAZBQLL-UHFFFAOYSA-N
應用
Synthesis of anti-inflammatory agents
Azide amidation
Reactions of thio acids with azides
Chemoselective sodium borohydride reduction of azides in water
Reagent for:
Photo-Stevens rearrangement
Cobalt-catalyzed synthesis of tertiary azides
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
商品
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门