推荐产品
品質等級
產品線
ReagentPlus®
化驗
≥99%
形狀
chips
crystalline powder
flakes
mp
108-110 °C (lit.)
溶解度
H2O: 50 mg/mL
儲存溫度
room temp
SMILES 字串
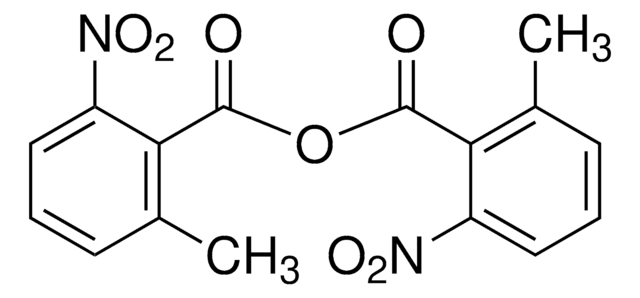
CN(C)c1ccncc1
InChI
1S/C7H10N2/c1-9(2)7-3-5-8-6-4-7/h3-6H,1-2H3
InChI 密鑰
VHYFNPMBLIVWCW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-二甲氨基吡啶(DMAP)是一种多功能亲核催化剂,用于酰化和酯化反应。它也可用于各种有机转化反应,如Baylis-Hillman反应、Dakin-West反应、胺的保护、C-酰化、硅烷化、天然产物化学中的应用。
應用
4-二甲氨基吡啶可用作以下反应的催化剂:
- 通过与丙二腈、醛和β-硝基烯反应合成3,5 取代的2,6-二氰基苯胺。
- 在无辅助碱和溶剂的条件下与酸酐进行醇的酰化反应合成相应的酯。
- 通过活化烯和醛或酮的偶联进行Baylis-Hillman 反应生成碳碳键。
酰化反应的高效催化剂
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 1
標靶器官
Nervous system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
255.2 °F
閃點(°C)
124 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Akira Iida et al.
Organic letters, 8(23), 5215-5218 (2006-11-03)
[Structure: see text] A powerful Ti-crossed Claisen condensation between ketene silyl acetals (KSAs) and acid chlorides was successfully performed to give alpha-monoalkylated esters and thermodynamically unfavorable (less accessible) alpha,alpha-dialkylated beta-keto esters in good yield (46 examples; 41-98% yield). A closely
A M van Wijk et al.
Analytical and bioanalytical chemistry, 400(5), 1375-1385 (2011-03-30)
A generic LC-MS/MS method was developed for the analysis of potentially genotoxic alkyl halides. A broad selection of alkyl halides were derivatized using 4-dimethylaminopyridine in acetonitrile. The reaction conditions for derivatization, i.e., solvent, reaction time, temperature and concentration of alkyl
Capture and visualization of hydrogen sulfide by a fluorescent probe.
Chunrong Liu et al.
Angewandte Chemie (International ed. in English), 50(44), 10327-10329 (2011-09-08)
Min Suk Shim et al.
Journal of controlled release : official journal of the Controlled Release Society, 133(3), 206-213 (2008-11-11)
To maximize therapeutic effects, targeted delivery of nucleic acids (e.g., DNA and RNA) in their appropriate intracellular targets is highly desirable. In this study, primary amines of a model polymeric nonviral carrier, polyethylenimine (PEI), at two molecular weights (0.8 and
Hangxiang Wang et al.
Journal of the American Chemical Society, 133(31), 12220-12228 (2011-07-19)
Catalysts hold promise as tools for chemical protein modification. However, the application of catalysts or catalyst-mediated reactions to proteins has only recently begun to be addressed, mainly in in vitro systems. By radically improving the affinity-guided DMAP (4-dimethylaminopyridine) (AGD) catalysts
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门






![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)