Long-Term Live Cell Tracking of Cancer and Stem Cells Using a Biocompatible Fluorescent Nanoparticle Based Upon Aggregation Induced Emission (AIE Dot) Nanotechnology
Nick Asbrock1, Kevin Su1, Vi Chu1, Bin Liu2
1Assay & Platform Development, Bioscience BU, EMD Millipore, 28820 Single Oak Drive, Temecula, CA, USA 92590, 2Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore
Introduction
Fluorescent imaging techniques with high spatial resolution, ease of access, and low cellular toxicities have been widely used for cell tracking and tracing experiments. Fluorescent reporters using Green/Red Fluorescent Protein (GFP/RFP) have dominated this field. However, they suffer from a number of drawbacks including: varied gene transfection efficiencies, gene mutation and poor photostability, which results in challenges for labelling and tracking of primary and stem cells. Luckily, the development of cell permeable dyes provided a simple direct fluorescent labelling approach. These fluorescent reagents are readily internalized into cells, are non-disruptive to normal cell activities and can be highly retained in cells during proliferation and differentiation. These labelling reagents possess extremely high initial fluorescence but are susceptible to signal quenching and photo-bleaching. Inorganic semiconductor quantum dots have recently been developed as cell labelling and tracking reagents. The quantum dot based trackers are highly emissive and photostable but the intrinsic heavy metal cores could lead to undesirable cytotoxicity, and the fluorescent blinking properties of quantum dots also limit their applications in ultra-fast scanning.
In this article, we highlight LuminiCell Tracker™ fluorescent nanoparticles for enhanced long-term visualizing and tracking of cancer and stems cells in both in vitro and in vivo environments. Different from conventional dyes, which suffer from concentration or aggregated caused quenching (ACQ), the LuminiCell trackers take advantage of novel dyes with aggregation-induced emission (AIE) properties1,2,3,4,5. These new AIE cell tracking dyes have been encapsulated into a cell permeable biocompatible fluorescent nanoparticles (AIE Dot); providing ultra-bright fluorescence (10X), enhanced photostability (3X) and low cellular toxicity over extended periods of time for bioimaging applications.
Figure 1. Physical characteristics of LuminiCell Tracker™ fluorescent nanoparticles.A) Fabrication of LuminiCell Tracker™ nanoparticles includes encapsulation of the fluorescent TPETPAFN AIE molecules within a DSPE-PEG200 outer shell with attached cell permeable TAT sequences; i) self-assembly and ii) bioconjugation. B) Physical stability comparison between LuminiCell Trackers™ and commercial Qtracker® when both were incubated in 1X PBS at 37 °C for 0 to 9 days.
Materials and Methods
Construction of LuminiCell Tracker™ 540 and 670
The fabrication of LuminiCell Tracker™ 540 or 670 requires three critical steps: design and synthesis of AIE dyes, construction of AIE dots, and surface functionalization. The AIE dye tetraphenylethene (TPE) is used for structure tuning and modification to provide the AIE dyes with green and red emission. Our established organic nanoparticle fabrication strategy is used to produce AIE Dot nanoparticles born with surface functional groups (Figure 1). Lastly, the synthesized AIE Dot nanoparticles with surface functionalities are further modified to ensure its cellular internalization and retention using TAT sequences.
Cell Labelling and Imaging
Cells in growth medium were seeded onto 8-well glass chamber for both fixed and live cell imaging. Seeding densities were selected to provide for 60 to 80% confluency after overnight culture. The next day after cell seeding, the growth medium was replaced with fresh growth medium containing diluted LuminiCell Tracker™ 540 or 670 with an incubation of 4 nM. The cells were then incubated at 37 ◦C, 5% CO2 incubator for 4 h. Different incubation concentration and time may be applied for different type cells. After 4 h incubation, the LuminiCell Tracker™ containing medium was removed and the cells were washed twice with 1 X PBS buffer, and the cells were ready for live cell visualization. For fixed cell imaging, the cells were fixed for 20 minutes at room temperature with 4% formaldehyde in PBS buffer. After 20 min fixation, the cells were rinsed twice with PBS buffer, and imaged.
Cell Tracking
Cells were cultured in 6-well plates in CO2 incubator at 37 oC with cell culture medium containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS). After overnight culturing, the cell culture medium was removed and cells were rinsed twice with 1XPBS. The labelling solution was prepared by diluting the stock LuminiCell Tracker™ solution using fresh cell culture medium with final concentration of 4 nM. 2 mL of labelling solution was added into each well. After 4 h incubation, the labelling solution was removed and the cells were washed twice with 1×PBS buffer. The cells were harvested by trypsin. The cell suspension were further centrifuged at 1500 rpm for 5 min and resuspended in fresh growth medium. The resuspended cells were diluted and subcultured into 6-well plates containing cell culture coverslips for designated generations. After designated time intervals, the selected cells were washed with PBS buffer and fixed by 75% ethanol or 3.7% formaldehyde in PBS for 20 minutes. The coverslips were sealed with mounting medium and the fluorescence images were studied by fluorescent microscope or confocal laser scanning microscope.
In vivo Tracking
For in vivo cell tracking demonstration, cancer cells after incubation with 4 nM LuminiCell Tracker™ for 4 h were harvested and resuspended in growth medium. The cell suspension (1 X 106 in 0.1 mL medium) was injected into the flank of mice. After designated time interval post injection, the mice were imaged by an IVIS® Spectrum Imaging system.
Results
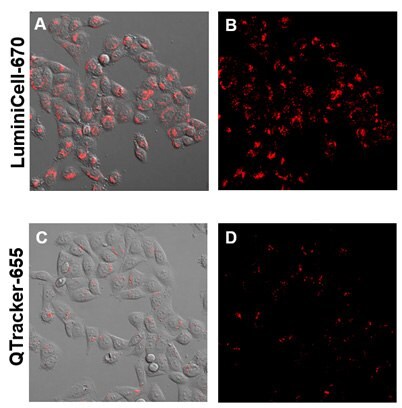
Figure 2. Live Cell imaging with LuminiCell Tracker™ nanoparticles.LuminiCell Trackers™ are non-toxic to cells and produce brighter fluorescence signals compared to traditional Quantum Dot based technologies. HeLa cells were plated at 500K cells per well of a 6-well plate overnight. On the next day, 4 nM LuminiCell Tracker™ 670 or Qtracker® 655 were added and incubated for 4 hours and then imaged at Day 1.
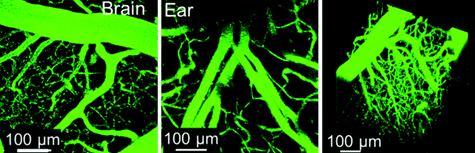
Figure 3. Vascular labelling experiments.The LuminiCell Tracker™-540 Vascular Labelling Kit allows for in vivo fluorescent blood vessel imaging in mouse brain and ear tissue. The kit contains green fluorescent AIE Dot nanoparticles without TAT sequences. These nanoparticles can be used to fluorescently tag vasculature in living tissues and animals for studies of inflammation and vascular leakage.
Figure 4. In Vitro Cancer Cell Tracking with LuminiCell Trackers™.To demonstrate the capability of the LuminiCell Tracker™-540 Cell Labeling Kit, HeLa cells were incubated with LuminiCell Tracker™ 540 at a concentration of 4 nM for 4 h, after which the incubation solution was discarded, and the cells were further subcultured for several days. The LuminiCell Tracker™ 540 showed very high labelling efficiency of nearly 100% at 1st day after incubation, which is still above 90% on the 5th day post-incubation. At 10th day post incubation, the distinguishable fluorescence from labelled cells could still be observed, indicating the excellent cell tracking ability of LuminiCell Tracker-540™ Cell Labeling Kit.
Figure 5. In Vivo Cancer Cell Tracking with LuminiCell Trackers™A) In vitro tracking of MCF-7 cancer cells with LuminiCell Tracker™-670 Cell Labeling Kit. B) Representative in vivo fluorescence image of mouse subcutaneously injected with 1 × 106 of MCF-7 cells immediately after labelling by 2 nM LuminiCell Tracker™ 670. C) The graph shows the integrated fluorescence intensity at the tumor sites over the total tracking period of 21 days.
Monitoring and understanding the fate of administrated stem cells in vivo is of great importance for stem cell therapies. A direct cell labelling technique appears to be the most promising technique, and in which, the LuminiCell Tracker™ with extremely high brightness, excellent photostability and generic staining ability is the ideal solution for staining and visualization of stem cell activities during stem cell therapy. Stem cell tracking was performed using LuminiCell Tracker™ 670 as the labelling reagent and adipose-derived mesenchymal stem cells (ADSCs). The in vitro tracking experiment revealed that the labelling rate was above 92.5% after 5 days in culture (Figure 6). Following implantation in a mouse, the fluorescence signal was still distinguishable in vivo at the transplanted site after 42 days (Figure 7A). Moreover, the single-cell co-staining analysis reveals that the LuminiCell Tracker™ 670 could remain inside ADSCs after 30 days (Figures 7B, 7C). This data supports the utility of the LuminiCell Trackers™ for extended cell tracking experiments.
Figure 6. In Vitro Stem Cell Tracking with LuminiCell Trackers™A) AIE dots labelling enables enhanced long-term tracking of adipose mesenchymal stem cells in vitro (A) ADSCs labelled with AIE dots, PKH26 as well as Qtracker® 655 and then subcultured for 1 and 5 days, respectively.
Figure 7. In Vivo Stem Cell Tracking with LuminiCell Trackers™A) In vivo long-term tracking of ADSCs. B) Confocal image of ischemic hind limb slice from mice after administration of LuminiCell Tracker™ 670 labelled ADSC-containing Matrigel for 30 days. C) Representative CD31-staining CLSM images of the ischemic hind limb slices from mice treated with LuminiCell Tracker™ 670 labelled ADSC-containing Matrigel for 42 days, the white arrows indicate the overlay yellow or orange fluorescence.
Conclusion
LuminiCell Tracker™ fluorescent nanoparticles provide a simple, safe, and effective reagent for long-term cell tracking experiments. LuminiCell is founded based on the novel technology of AIE, which takes the advantage of naturally unpreventable aggregate to provide labelling reagents with ultra-high brightness. LuminiCell Trackers™ show superior fluorescence quantum yield, high resistance to photo-bleaching and excellent biocompatibility, which works with most fluorescence microscopy and flow cytometry units. The data presented here demonstrate the capability of LuminiCell Tracker for in vitro and in vivo tracking of cell fate and activities by using cancer cells and ADSCs as models, which have shown superior tracking time which cannot be achieved by other direct fluorescence labelling techniques.
References
To continue reading please sign in or create an account.
Don't Have An Account?