SAB4200609
Monoclonal Anti-diMethyl-Histone H3 (diMe-Lys9) (H3K9me2) antibody produced in mouse
~1.0 mg/mL, clone 5E5-G5, purified immunoglobulin
Synonym(s):
Anti-H3F3A H3.3A H3F3 PP781 H3F3B H3.3B, Anti-HIST1H3A H3FA, Anti-HIST1H3B H3FL, Anti-HIST1H3C H3FC, Anti-HIST1H3D H3FB, Anti-HIST1H3E H3FD, Anti-HIST1H3F H3FI, Anti-HIST1H3G H3FH, Anti-HIST1H3H H3FK, Anti-HIST1H3I H3FF, Anti-HIST1H3J H3FJ, Anti-HIST2H3A HIST2H3C H3F2 H3FM HIST2H3D
About This Item
Recommended Products
biological source
mouse
Quality Level
conjugate
unconjugated
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
5E5-G5, monoclonal
form
buffered aqueous solution
mol wt
antigen ~17 kDa
concentration
~1.0 mg/mL
technique(s)
immunoblotting: 2-4 μg/mL using histones isolated from human HeLa cells.
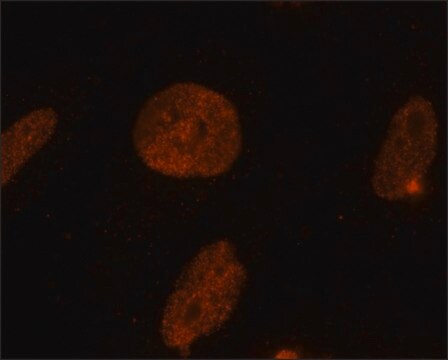
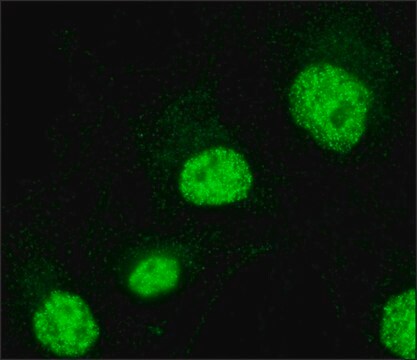
immunofluorescence: 1-2 μg/mL using human HeLa cells.
isotype
IgG1
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
dimethylation (Lys9)
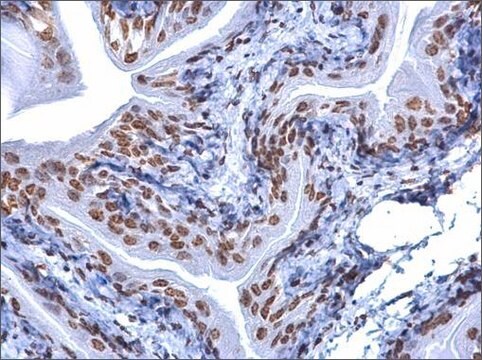
General description
Immunogen
Application
Biochem/physiol Actions
Physical form
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class
10 - Combustible liquids
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| SAB4200609-200UL | 4061838037121 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service