O4878
Oxaloacetate Decarboxylase from Pseudomonas sp.
lyophilized powder, ≥100 units/mg solid
Synonym(s):
OAD
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
form
lyophilized powder
specific activity
≥100 units/mg solid
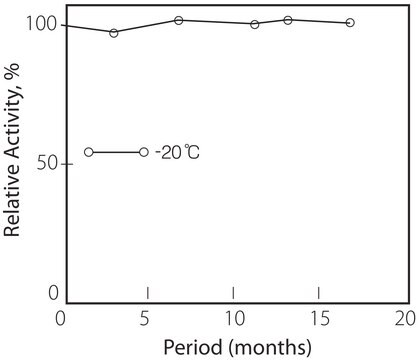
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Oxaloacetate decarboxylase or OAD functions as a Na pump in anaerobic bacteria. It is a membrane protein consisting of three subunits, α, β and γ with the α subunit containing the carboxylase activity.
Application
Oxaloacetate Decarboxylase from Pseudomonas sp. has been used in the digestion of the low molecular weight (LMW) human milk fraction (5kF fraction) and as a positive control for deciphering C. thermocellum oxaloacetate decarboxylase activity.
Oxaloacetate decarboxylase has been used in a study to assess turnover and accessibility of a reentrant loop of the Na(+)-glutamate transporter GltS. It has also been used in a study to investigate fermentation and metabolic characteristics of Gluconacetobacter oboediens for different carbon sources.
Biochem/physiol Actions
Oxaloacetate Decarboxylase catalyzes the decarboxylation of oxaloacetate and requires manganese and magnesium for its activity. It is associated with a wide vareity of Gram-negative bacteria.
Unit Definition
One unit will convert 1.0 μmole of oxalacetate to pyruvate and CO2 per min at pH 8.0 at 25 °C.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pius Dahinden et al.
Archives of microbiology, 182(5), 414-420 (2004-10-19)
Archaeoglobus fulgidus harbors three consecutive and one distantly located gene with similarity to the oxaloacetate decarboxylase Na+ pump of Klebsiella pneumoniae (KpOadGAB). The water-soluble carboxyl transferase (AfOadA) and the biotin protein (AfOadC) were readily synthesized in Escherichia coli, but the
Y Augagneur et al.
Journal of applied microbiology, 104(1), 260-268 (2007-10-12)
Citrate metabolism generates metabolic energy through the generation of a membrane potential and a pH gradient. The purpose of this work was to study the influence of oxaloacetate decarboxylase in citrate metabolism and intracellular pH maintenance in relation to acidic
Buvaneswari C Narayanan et al.
Biochemistry, 47(1), 167-182 (2007-12-18)
Pseudomonas aeruginosa PA4872 was identified by sequence analysis as a structurally and functionally novel member of the PEP mutase/isocitrate lyase superfamily and therefore targeted for investigation. Substrate screens ruled out overlap with known catalytic functions of superfamily members. The crystal
Dayanidhi Sarkar et al.
Applied microbiology and biotechnology, 87(1), 127-136 (2010-03-02)
The metabolism of Gluconacetobacter oboediens was investigated in relation to different carbon sources for the continuous cultures at the dilution rate of 0.05 h(-1). The 13C-flux result implies the formation of metabolic recycles for the case of using glucose and
Pius Dahinden et al.
Archives of microbiology, 183(2), 121-129 (2005-01-14)
The oxaloacetate decarboxylase (OAD) Na(+) pump consists of subunits alpha, beta, and gamma, which are expressed from an oadGAB gene cluster present in various anaerobic bacteria. Vibrio cholerae has two copies of oad genes, which are termed oad-1 and oad-2.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service