O104
Omeprazole
≥98% (HPLC), solid, gastric secretion inhibitor
Synonym(s):
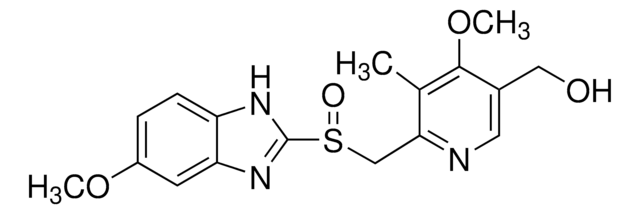
5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, Antra, Losec
About This Item
Recommended Products
Product Name
Omeprazole, solid
form
solid
color
white
solubility
H2O: 0.5 mg/mL
DMSO: >19 mg/mL
ethanol: 4.5 mg/mL
originator
AstraZeneca
storage temp.
2-8°C
SMILES string
COc1ccc2[nH]c(nc2c1)S(=O)Cc3ncc(C)c(OC)c3C
InChI
1S/C17H19N3O3S/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)
InChI key
SUBDBMMJDZJVOS-UHFFFAOYSA-N
Gene Information
human ... ABCB1(5243) , ATP4A(495) , ATP4B(496) , CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Features and Benefits
Caution
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Discover Bioactive Small Molecules for ADME/Tox
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service