MTOX1020
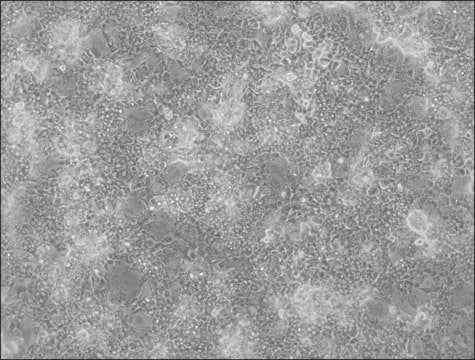
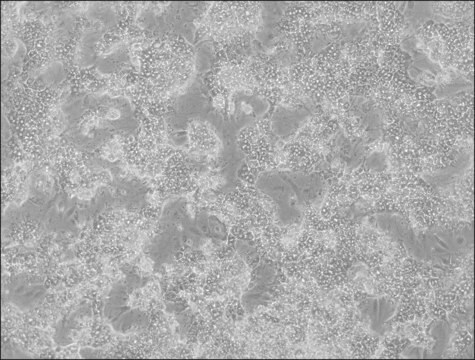
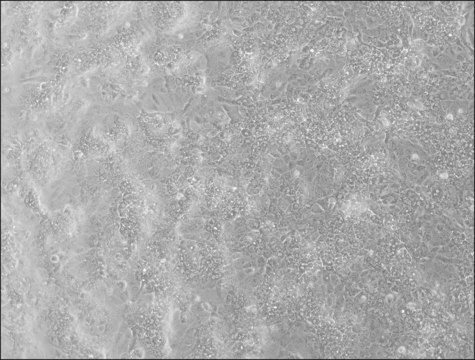
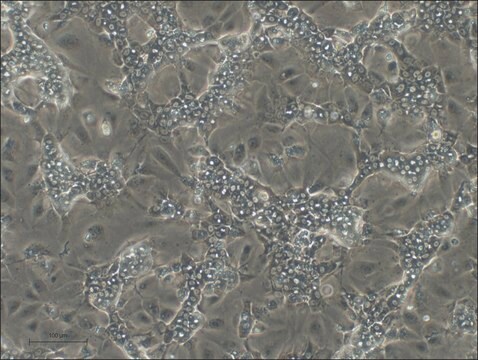
HepaRG™ Cells MRP2 (-/-)
human female liver (hepatocarcinoma and hepatitis C tumor)
About This Item
Recommended Products
product name
HepaRG™ Cells MRP2 (-/-), one vial
biological source
human female liver (hepatocarcinoma and hepatitis C tumor)
form
liquid
OMIM accession no.
storage temp.
−196°C
Gene Information
human ... ABCC2(1244)
General description
Application
Features and Benefits
- The frame-shift mutation of ABCC2 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
Quality
Legal Information
Exhibit 2: HepaRG limited use license
Disclaimer
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service