G6920
Endo-β-galactosidase from Bacteroides fragilis
recombinant, expressed in E. coli, ≥140 units/mg protein, buffered aqueous solution
Synonym(s):
β-Galactosidase bacterial, Keratanase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli
Quality Level
conjugate
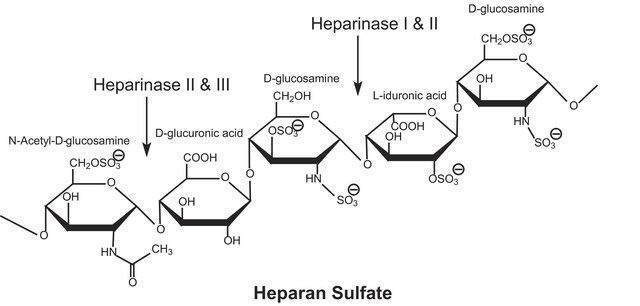
(Glucosaminoglycan)
sterility
aseptically filled
form
buffered aqueous solution
specific activity
≥140 units/mg protein
mol wt
32 kDa
storage temp.
2-8°C
Related Categories
Application
Endo-β-galactosidase was used in fractional protein isolation. It was used for deglycosylation in glycoproteomics of the endothelial secretome of human endothelial cells.
Biochem/physiol Actions
Internal β(1-4) galactose linkages in unbranched, repeating poly-Nacetyllactosamine [GlcNAc β(1-3)Gal β (1-4)] structures are the preferred substrate.
Sulfated structures such as keratan sulfate are also cleaved. Branching and/or fucosylation of the substrate may reduce or completely inhibit cleavage. Sulfation of C-6 on galactose will block cleavage. Oligosaccharides of the neolacto-group are cleaved at greatly reduced rates depending on the deviation from the preferred substrate. For example, Gal β(1-3)GlcNAc β(1-3) Gal β(1-4)Glc is cleaved at 5X10-5 the rate of keratan sulfate
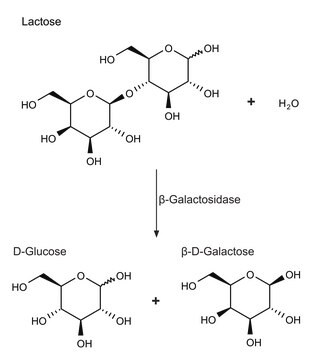
β-galactosidase cleaves lactose into its monosaccharide components, glucose and galactose. It also catalyses the transglycosylation of glucose into allolactose, the inducer of β-galactosidase, in a feedback loop.
Sulfated structures such as keratan sulfate are also cleaved. Branching and/or fucosylation of the substrate may reduce or completely inhibit cleavage. Sulfation of C-6 on galactose will block cleavage. Oligosaccharides of the neolacto-group are cleaved at greatly reduced rates depending on the deviation from the preferred substrate. For example, Gal β(1-3)GlcNAc β(1-3) Gal β(1-4)Glc is cleaved at 5X10-5 the rate of keratan sulfate
β-galactosidase cleaves lactose into its monosaccharide components, glucose and galactose. It also catalyses the transglycosylation of glucose into allolactose, the inducer of β-galactosidase, in a feedback loop.
Unit Definition
One unit will release 1.0 μmole of reducing sugar from bovine corneal keratan sulfate per minute at 37 °C, pH 5.8.
Physical form
Aseptically filled solution in 20 mM Tris-HCl, pH 7.5
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maria Vistnes et al.
PloS one, 9(3), e89621-e89621 (2014-03-07)
We hypothesized that cleavage of the extracellular matrix (ECM) proteoglycans versican and aggrecan by ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) proteases, which contributes to stress-induced ECM-reorganization in atherogenesis and osteoarthritis, also play a role in heart failure development.
Xiaoke Yin et al.
Molecular & cellular proteomics : MCP, 12(4), 956-978 (2013-01-25)
Previous proteomics studies have partially unraveled the complexity of endothelial protein secretion but have not investigated glycosylation, a key modification of secreted and membrane proteins for cell communication. In this study, human umbilical vein endothelial cells were kept in serum-free
William Mark Erwin et al.
Arthritis research & therapy, 17, 240-240 (2015-09-06)
In the present study, we sought to quantify and contrast the secretome and biomechanical properties of the non-chondrodystrophic (NCD) and chondrodystrophic (CD) canine intervertebral disc (IVD) nucleus pulposus (NP). We used iTRAQ proteomic methods to quantify the secretome of both
Salvatore Santamaria et al.
Scientific reports, 9(1), 10914-10914 (2019-07-31)
ADAMTS (A Disintegrin-like and Metalloproteinase domain with Thrombospondin type 1 Motif)-1, -4 and -5 share the abilities to cleave large aggregating proteoglycans including versican and aggrecan. These activities are highly relevant to cardiovascular disease and osteoarthritis and during development. Here
Xiaoke Yin 殷晓科 et al.
Arteriosclerosis, thrombosis, and vascular biology, 39(9), 1859-1873 (2019-07-19)
Marfan syndrome (MFS) is caused by mutations in FBN1 (fibrillin-1), an extracellular matrix (ECM) component, which is modified post-translationally by glycosylation. This study aimed to characterize the glycoproteome of the aortic ECM from patients with MFS and relate it to
Articles
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service