G6125
Glucose Oxidase from Aspergillus niger
Type II, ≥10,000 units/g solid (without added oxygen)
Synonym(s):
β-D-Glucose:oxygen 1-oxidoreductase, G.Od., GOx
About This Item
Recommended Products
type
Type II
form
powder
specific activity
≥10,000 units/g solid (without added oxygen)
mol wt
160 kDa
foreign activity
amylase ≤0.5%
catalase ≤2 Sigma units/mg solid
galactose oxidase ≤3%
glycogenase ≤0.5%
invertase ≤0.5%
maltase ≤2%
storage temp.
−20°C
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6-/m1/s1
InChI key
WQZGKKKJIJFFOK-VFUOTHLCSA-N
Looking for similar products? Visit Product Comparison Guide
General description
pI: 4.2
Extinction coefficient: E1% = 16.7 (280 nm)
Glucose oxidase from Aspergillus niger is a dimer consisting of 2 equal subunits with a molecular mass of 80 kDa each. Each subunit contains one flavin adenine dinulceotide moiety and one iron. The enzyme is a glycoprotein containing ~16% neutral sugar and 2% amino sugars. The enzyme also contains 3 cysteine residues and 8 potential sites for N-linked glycosylation.
Glucose oxidase is capable of oxidizing D-aldohexoses, monodeoxy-D-glucoses, and methyl-D-glucoses at varying rates.
The pH optimum for glucose oxidase is 5.5, while it has a broad activity range of pH 4-7. Glucose oxidase is specific for β-D-glucose with a KM of 33-110 mM.
Glucose oxidase does not require any activators, but it is inhibited by Ag+, Hg2+, Cu2+, phenylmercuric acetate, and p-chloromercuribenzoate. It is not inhibited by the nonmetallic SH reagents: N-ethylmaleimide, iodoacetate, and iodoacetamide.
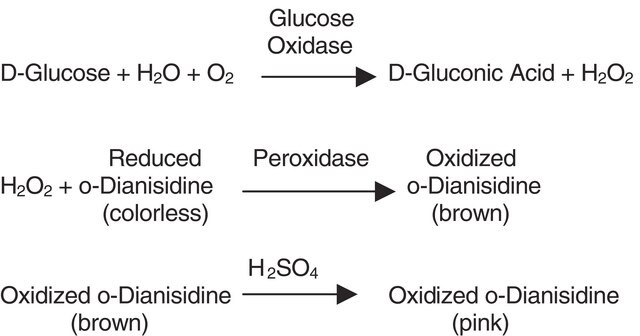
Glucose oxidase can be utilized in the enzymatic determination of D-glucose in solution. As glucose oxidase oxidizes β-D-glucose to D-gluconolactate and hydrogen peroxide, horseradish peroxidase is often used as the coupling enzyme for glucose determination. Although glucose oxidase is specific for β-D-glucose, solutions of D-glucose can be quantified as α-D-glucose will mutorotate to β-D-glucose as the β-D-glucose is consumed by the enzymatic reaction.
Application
Biochem/physiol Actions
Caution
Linkage
Unit Definition
Analysis Note
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service