G1549
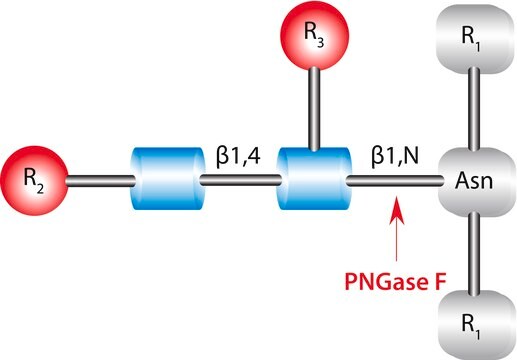
PNGase F from Elizabethkingia meningoseptica
ready-to-use solution, recombinant, expressed in E. coli
Synonym(s):
PNGase F from Elizabethkingia meningoseptica, N-Glycosidase F, PNGase F from Chryseobacterium meningosepticum, PNGase F from Flavobacterium meningosepticum, Peptide N-glycosidase
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
conjugate
(N-linked)
grade
Proteomics Grade
form
ready-to-use solution
specific activity
≥1000 U/mg
shelf life
≥1 yr at -20 °C
mol wt
~36 kDa
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Unit Definition
Physical form
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
N-Linked Glycan Strategies.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service