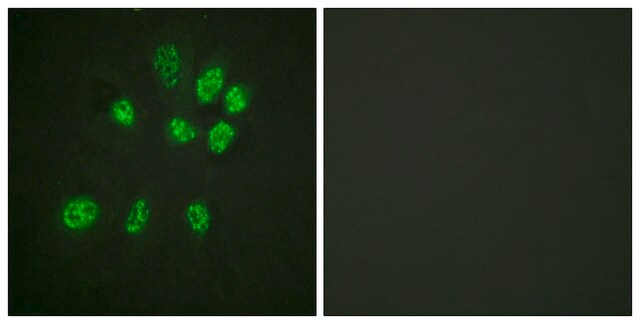
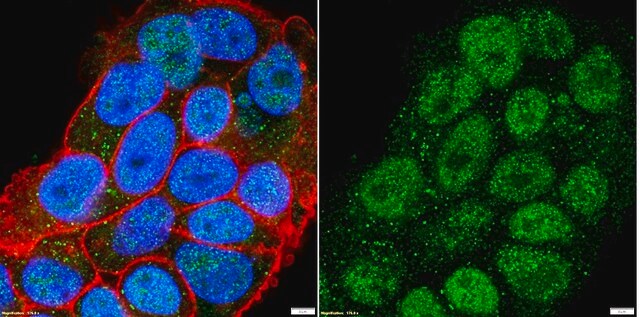
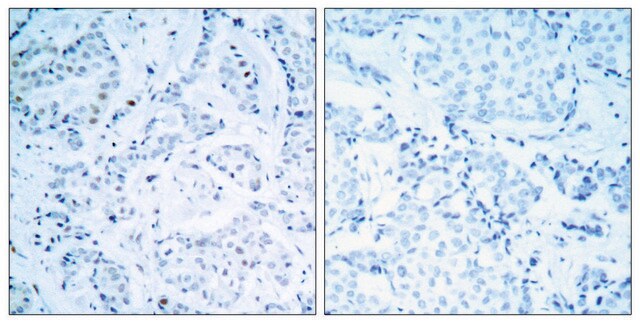
E1528
Estrogen Receptor-α human
≥80% (SDS-PAGE), recombinant, expressed in baculovirus infected insect cells, buffered aqueous glycerol solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in baculovirus infected insect cells
Quality Level
assay
≥80% (SDS-PAGE)
form
buffered aqueous glycerol solution
mol wt
66.4 kDa
packaging
vial of 211 pmol
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... ESR1(2099)
Biochem/physiol Actions
Hormone-inducible transcription factor capable of acting positively or negatively in regulating genes involved in tissue growth and differentiation. For use in signal transduction, steroid biochemistry, and endocrine disruptor research.
Physical form
Solution in 50 mM Tris-HCl, pH 8.0, 500 mM KCl, 2 mM DTT, 1 mM EDTA, 1 mM orthovanadate, and 10% glycerol.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G G Kuiper et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(12), 5925-5930 (1996-06-11)
We have cloned a novel member of the nuclear receptor superfamily. The cDNA of clone 29 was isolated from a rat prostate cDNA library and it encodes a protein of 485 amino acid residues with a calculated molecular weight of
G G Kuiper et al.
Endocrinology, 138(3), 863-870 (1997-03-01)
The rat estrogen receptor (ER) exists as two subtypes, ER alpha and ER beta, which differ in the C-terminal ligand binding domain and in the N-terminal transactivation domain. In this study we investigated the messenger RNA expression of both ER
K Paech et al.
Science (New York, N.Y.), 277(5331), 1508-1510 (1997-09-05)
The transactivation properties of the two estrogen receptors, ERalpha and ERbeta, were examined with different ligands in the context of an estrogen response element and an AP1 element. ERalpha and ERbeta were shown to signal in opposite ways when complexed
S Mosselman et al.
FEBS letters, 392(1), 49-53 (1996-08-19)
A novel estrogen receptor (hereinafter referred to as ER beta) was cloned using degenerate PCR primers. A comparison of the amino acid sequence of ER beta with the "classical' ER (ER alpha) shows a high degree of conservation of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service