85990
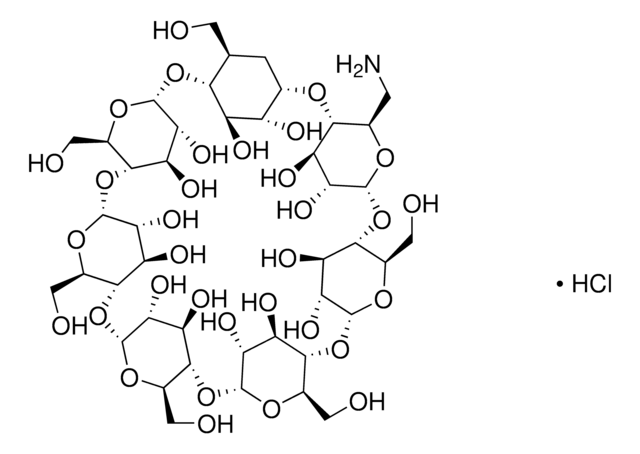
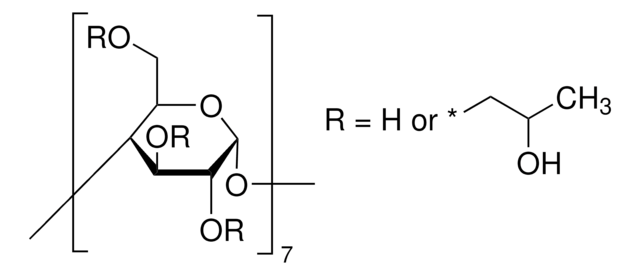
Succinyl-β-cyclodextrin
Synonym(s):
succ-β-CD, succinyl-β-CD
About This Item
Recommended Products
form
powder
impurities
~5% water
color
white
mp
225 °C ((437 °F ) - Decomposes on heating)
storage temp.
−20°C
SMILES string
CC(=O)CCC(=O)OC[C@H]1O[C@@H]2O[C@H]3[C@@H](O)[C@H](O)[C@H](O[C@@H]3COC(=O)CCC(O)=O)O[C@H]4[C@@H](O)[C@H](O)[C@H](O[C@@H]4COC(=O)CCC(O)=O)O[C@H]5[C@@H](O)[C@H](O)[C@H](O[C@@H]5COC(=O)CCC(O)=O)O[C@H]6[C@@H](O)[C@H](O)[C@H](O[C@@H]6COC(=O)CCC(O)=O)O[C@H]7[C@@H](O)[C@H](O)[C@H](O[C@@H]7COC(=O)CCC(O)=O)O[C@H]8[C@@H](O)[C@H](O)[C@H](O[C@@H]8COC(=O)CCC(O)=O)O[C@H]1[C@@H](O)[C@@H]2O
InChI
1S/C71H100O55/c1-23(72)2-9-37(85)106-16-24-58-44(92)51(99)65(113-24)121-59-25(17-107-38(86)10-3-31(73)74)115-67(53(101)46(59)94)123-61-27(19-109-40(88)12-5-33(77)78)117-69(55(103)48(61)96)125-63-29(21-111-42(90)14-7-35(81)82)119-71(57(105)50(63)98)126-64-30(22-112-43(91)15-8-36(83)84)118-70(56(104)49(64)97)124-62-28(20-110-41(89)13-6-34(79)80)116-68(54(102)47(62)95)122-60-26(18-108-39(87)11-4-32(75)76)114-66(120-58)52(100)45(60)93/h24-30,44-71,92-105H,2-22H2,1H3,(H,73,74)(H,75,76)(H,77,78)(H,79,80)(H,81,82)(H,83,84)/t24-,25-,26-,27-,28-,29-,30-,44+,45+,46+,47+,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-/m1/s1
InChI key
DIRLEDPEXJLCIL-JCWBWLHSSA-N
General description
Application
Succinyl-β-cyclodextrin and carboxymethyl-β-cyclodextrin are used as chiral selective agents in capillary electrophoresis for the separation of di- and tri-peptide enantiomers and catechin enantiomers. Succinyl-β-cyclodextrin is used to optimize analysis of PNA-DNA duplexes with diethylthiadicarbocyanine dye.
- Acylamine fungicides in commercial agrochemical formulations by electrokinetic chromatography (EKC).
- Catechin isomers in human biological samples and antihistamines in pharmaceutical preparations by CE.
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service