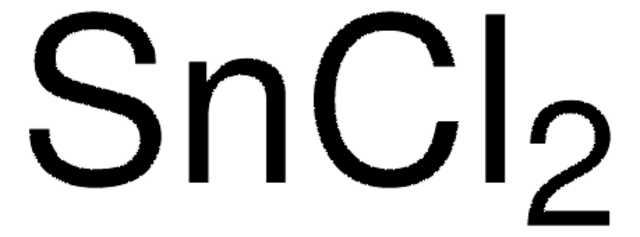208035
Tin(II) chloride dihydrate
reagent grade, 98%
Synonym(s):
Stannous chloride dihydrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
SnCl2 · 2H2O
CAS Number:
Molecular Weight:
225.65
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
reagent grade
Quality Level
assay
98%
form
powder, crystals or chunks
bp
652 °C (lit.)
mp
37-38 °C (dec.) (lit.)
solubility
water: soluble
storage temp.
2-8°C
SMILES string
O.O.Cl[SnH2]Cl
InChI
1S/2ClH.2H2O.Sn/h2*1H;2*1H2;/q;;;;+2/p-2
InChI key
FWPIDFUJEMBDLS-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Crystals of SnCl2.2H2O exhibit monoclinic crystal system and space group P21/c.
Application
Tin(II) chloride dihydrate (SnCl2.2H2O)may be used in the preparation of indoles. It may be used in the transformation of conjugated dioxolones, conjugated and non-conjugated acetals (dimethoxy and diethoxy acetals) to aldehydes.
It plays the role of a catalyst in the synthesis of 3-aminoimidazo[1,2-a]pyridines. It can be used as precursor for the synthesis of tin dioxide (SnO2) nanostructures by thermal degradation at temperature between 400-700 under controlled conditions.
It plays the role of a catalyst in the synthesis of 3-aminoimidazo[1,2-a]pyridines. It can be used as precursor for the synthesis of tin dioxide (SnO2) nanostructures by thermal degradation at temperature between 400-700 under controlled conditions.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An X-Ray Redetermination of the Crystal Structure of Tin (II) Chloride Dihydrate.
Kiriyama H, et al.
Bulletin of the Chemical Society of Japan, 46(5), 1389-1395 (1973)
Tin (II) chloride dihydrate: A mild and efficient reagent for cleaving acetals.
Ford KL and Roskamp EJ.
Tetrahedron Letters, 33(9), 1135-1138 (1992)
Tin (II) Chloride Dihydrate Catalyzed Groebke Condensation: An Efficient Protocol for the Synthesis of 3-Aminoimidazo[1,2-a]pyridines.
Shaabani A, et al.
Chin. J. Chem., 27(2), 369-371 (2009)
Ruthenium-catalysed synthesis of indoles from anilines and trialkanolamines in the presence of tin (II) chloride dihydrate.
Cho CS.
Chemical Communications (Cambridge, England), 9, 995-996 (1998)
Optical properties of SnO2 nanostructures prepared via one-step thermal decomposition of tin (II) chloride dihydrate.
Al-Gaashani R, et al.
Materials Science and Engineering, B, 177(6), 462-470 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service