All Photos(5)
About This Item
Empirical Formula (Hill Notation):
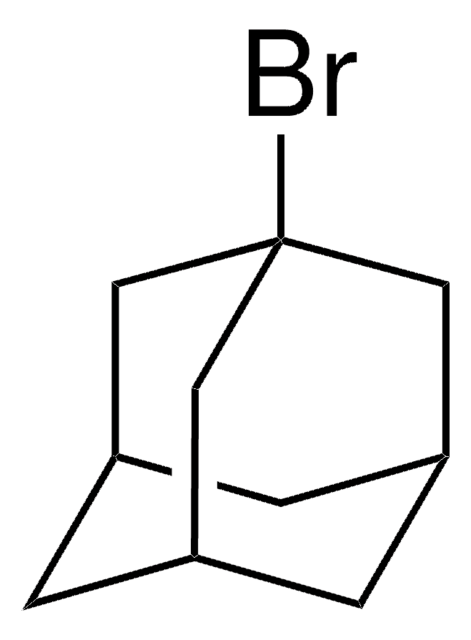
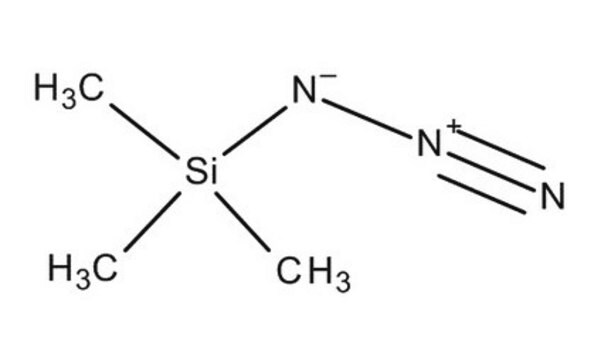
C10H15N3
CAS Number:
Molecular Weight:
177.25
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
assay
97%
form
powder or crystals
technique(s)
titration: suitable
mp
80-82 °C (lit.)
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
2-8°C
SMILES string
[N-]=[N+]=NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C10H15N3/c11-13-12-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6H2/t7-,8+,9-,10-
InChI key
JOMZSYXWYOVFEE-CHIWXEEVSA-N
Application
Building block for "Anti-Bredt"strained molecules.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Krzysztof Matyjaszewski et al.
Molecules (Basel, Switzerland), 26(2) (2021-01-14)
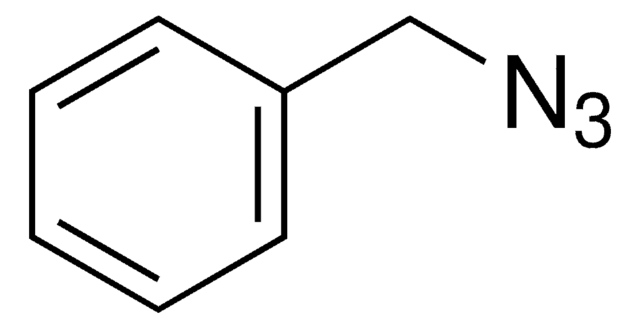
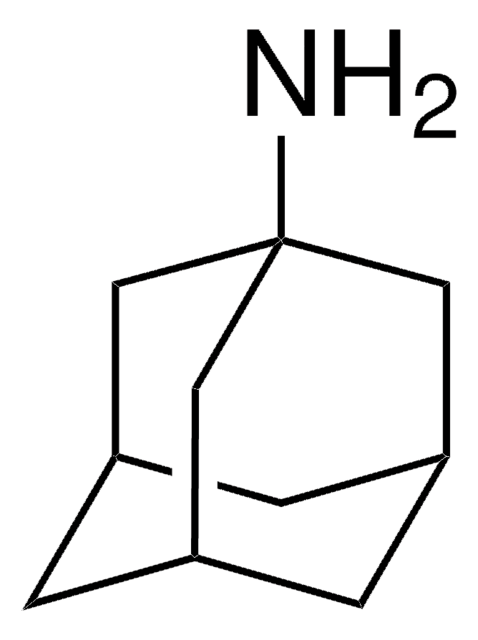
N-alkyl phosphoranimines were synthesized via the Staudinger reaction of four different alkyl azides with tris(2,2,2-trifluoroethyl) phosphite. N-adamantyl, N-benzyl, N-t-butyl, and N-trityl phosphoranimines were thoroughly characterized and evaluated as chain-capping compounds in the anionic polymerization of P-tris(2,2,2-trifluoroethoxy)-N-trimethylsilyl phosphoranimine monomer. All four
Aldrichimica Acta, 18, 82-82 (1985)
Atif Sarwar et al.
PloS one, 10(4), e0123084-e0123084 (2015-05-01)
Recently, the attention of researchers has been drawn toward the synthesis of chitosan derivatives and their nanoparticles with enhanced antimicrobial activities. In this study, chitosan derivatives with different azides and alkyne groups were synthesized using click chemistry, and these were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service